| (Clicking
on the thumbnail images will launch a new window and a larger version
of the thumbnail.) |
| Last
updated: July 10, 2004. |
Cavia porcellus
by Peter Kaufmann
Order: Rodentia
Family: Caviidae
1) General Zoological Data
The guinea pig is the best known representative of caviomorph rodents. This is probably due to the fact that it became the only truly domesticated caviomorph. This small South American rodent is considered to be a delectable meal for South American natives and, therefore, it was kept and bred as domestic animal long before becoming largely extinct in the wild. Moreover, in the 20th century it started its triumph around the world as pet. Cavia porcellus is the domestic species and was derived from its wild ancestor Cavia aperea. The sporadic use of the designation Cavia aperea porcellus may point to this origin, but also to existing hybrids (Cavia aperea x Cavia porcellus). The name "guinea" is mistaken; it should have been Guyana, its origin; "cavia" is a Tupi word for rat, and "porcellus" refers to little pig (Gotch, 1979).
With the exception of the human placenta, the guinea pig placenta is today the best studied placenta. The reasons are twofold. First, the guinea pig became one of the favorite experimental animals for pharmacologists and toxicologists, so that basic data concerning its reproduction were essential and asked for. Second, already the first examinations of its placenta by Enders (1965), Vollrath (1965), Müller & Fischer (1968), Kaufmann (1969), and Davidoff & Schiebler (1970) made it clear that (a) due to a series of similarities with the human placenta and (b) because of several advantages over other experimental models, such as the widely-used sheep, the guinea pig might become a perfect animal model for placentologists. The guinea pig, different from other laboratory rodents, has a rather long gestation period (up to 70 days); it is handy, patient and easily bred; it has an endocrine pregnancy control similar to that of the human, and it has a discoidal, hemomonochorial placenta with a fetal/maternal transport barrier which is very similar to that of the human placenta. Consequently, for a period of three decades, the guinea pig started to replace the sheep and became the favorite model in placentology. The success of this animal model has ceased only recently because of the explosively developing human in vitro models, such as primary cell cultures isolated from the human placenta, immortal placental cell lines, placental tissue explants and human placental lobules perfused in vitro. Longevity of domestic guinea pigs is up to six years.
There is a very large variety of guinea pig strains, coat color etc. They are easily accessible through various web sites. These also provide information on diseases, home care, length of expected fertility (4-5 litters), possible problems in assigning sex, and many more topics. One useful site is: http://www.oginet.com/pgurney/. The picture shown comes from another such sites (http://www.meerschweinchen.de by K. Stuber, Germany).
| Domestic guinea pig (Abyssinian tortoiseshell). | |
Length of gestation: 63 - 70 days
Litter size: 1 - 9, mean 4
Body weight (non pregnant): 750 g, at full term: 1,000 - 1,400 g
Fetal weight at full term: 60 - 100 g
Fetal crown-rump length at full term: mean 100 mm
Weight of placenta and membranes at full term: 5 - 9 g
Organ weight data may be found in the contribution by Webster & Liljegren (1949).
With respect to litter size and gestational length, the experimentator should be aware of some important facts: In wild guinea pigs and in domestic breeds of the sixties, the litter size was rather low (2.3) and gestational length rather short (59 to 63 days) (Weir, 1974; Kaufmann, 1969); in contrast, conventional domestic breeds and inbred strains developed later in the same century tended to produce larger litter sizes and showed an increase in pregnancy length together with an increase in fetal and placental weights; in the meantime, some inbred strains (e.g. Pirbright-White) produce litter sizes up to 12 (mean >6) and have gestational lengths of >72 days. There is also evidence that litter size, gestational length, fetal and placental weight at term increase with increasing age, parity and body weight of the sow (Kaufmann & Davidoff, 1977). For experimental purposes, it is therefore highly advisable to work with colonies that are as homogeneous as possible regarding strain, maternal body weight, parity and age of the female animals.
3) Implantation
Guinea pigs have an interstitial, antimesometrial implantation between
days 5 and 6 post conception. The early stages of fetal, placental and
yolk sac development in the guinea pig are very complex and involve some
mechanisms specific to caviomorph rodents (e.g. yolk sac involution, development
of a subplacenta). These processes were described in much detail by Duval
as early as in 1892. Brief diagrammatic summaries can be found in Kaufmann
& Davidoff (1977) and Uhlendorf & Kaufmann (1979).
The general placental type is discoidal, labyrinthine, hemo-monochorial that represent the chorioallantoic main placenta, and with separate subplacenta and yolk sac placenta (Kaufmann & Davidoff, 1977). The allantois is only used for the formation and vascularization of the chorioallantoic placenta (main placenta and subplacenta).
As far as the gross anatomy of the fetal membranes is concerned, the guinea pig has two uterine horns, each horn providing space for one to five implantation sites. The elongated uterine horns unite to form a short, common corpus uteri. Each fetus is surrounded by (Fig. 1) an inner fetal membrane, the amnion (Figures 3a, b), and an outer fetal membrane, which is represented over 95% of its surface (antimesometrially and laterally) by the visceral yolk sac (Figures 3a,b); and only at about 5% of its surface (mesometrially) it is made up by the chorioallantoic main placenta, a small disc-like organ (Figures 3a,b); at its basis the latter has a stem-like connection to the uterine wall, the placental stalk, which contains the subplacenta, representing a special segment of the chorioallantoic placenta (Figures 2, 3a,b).
The gross anatomy of the chorioallantoic main placenta is represented by the main fetal/maternal exchange organ, the disc. As in all caviomorph rodents, it consists of a lens-like disc of syncytiotrophoblast (Figure 2), which is traversed throughout by a system of web-like channels containing maternal blood, the maternal blood lacunae. Additionally it is traversed only in its more central sections by fetal capillaries.
Miglino et al. (2004) have presented a remarkable comparative study of the placentas of agouti, capybara, guninea pig, paca and rock cavy that gives superb details of the vascular organization of these organs.
The parts perfused by both, maternal and fetal blood channels, make up the so-called labyrinth. It is composed of 60 to 100 cylindrically shaped structures, the labyrinthine lobes which vertically pass the placental disc (Below). They make up about 80% of the main placental volume at term. The lobes measure between 1 and 2 mm in diameter, and 5 to 8 mm in length. All lobes are connected to each other close to their fetal ends. The labyrinth provides the vast majority of fetal/maternal exchange tissue of the guinea pig placenta.
The labyrinthine lobes are embedded into syncytiotrophoblast which is only maternally perfused, the so-called interlobium (about 15% of the main placental volume at term). The interlobium separates the labyrinthine lobes from each other ('interlobar syncytium') and forms the outer mantle of the main placental disc ('marginal syncytium'). The maternal blood lacunae of the interlobium provide the venous blood channels that drain the labyrinth of maternal blood. The syncytiotrophoblast lining the maternal blood lacunae is the secretory source of the progesterone-binding protein (PBP) (Perrot Applanat & David Ferreira, 1982).
In its center, the main placenta is passed by an axis of fetally vascularized mesenchyme, the central excavation (about 5% of the main placental volume at term). This is the continuation of the umbilical cord, across the main placenta, down to the subplacenta. At its basal end, the central excavation widens like an umbrella ('roof of the central excavation'). Those parts of the chorioallantoic placenta, basal to the roof of the central excavation, by definition are designated subplacenta (see section 11 below).
| Figure 4a,b : Macroscopic lobules of guinea pig placenta. | |
The labyrinthine lobes represent the dominating part of the fetal/maternal exchange zone. In caviomorph rodents this is an ideal labyrinthine, hemomonochorial placenta with counter-current arrangement of blood vessels.
Labyrinthine in this context means: a lens-like mass of syncytiotrophoblast (the placental disc) (shown below) is passed, like in Swiss cheese, by a web-like system of channels, half of which contain maternal blood lacunae, which have no own endothelial vessel walls; and the remaining ones contain fetal capillaries, lined by fetal endothelium which is separated from the syncytiotrophoblast by a basal lamina.
 |
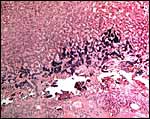
Labyrinthine portion of guinea pig placenta at term. |
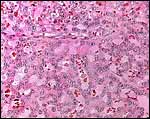 |
Higher magnification of labyrinth. |
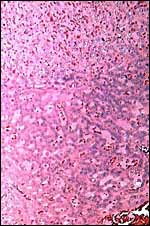 |
The edge of the labyrinth. |
| Figure 4c: Labyrinthine portion traversed by maternal channels. | |
- an uninterrupted layer of syncytiotrophoblast, beneath which, but only in the first half of gestation, accidental trophoblast cells (cytotrophoblast) can be found;
- a basal lamina, jointly secreted by syncytiotrophoblast and endothelium;
- few connective tissue cells, mostly macrophages, and very few accompanying collagen fibers;
- fetal endothelium which only in the center of the lobes may be accompanied by accidental pericytes.
The counter-current arrangement of vessels (Figure 7) in this context means: Both,
- the maternal blood lacunae (blood flow direction from the center of the cylindrically shaped lobes towards their periphery),
- and the fetal capillaries (blood flow direction from the surface of the lobes towards their centers), are arranged in such a way (Figures 7, 8) that maternal and fetal blood circulates in parallel but opposite directions. According to Faber & Hart (1966) this is the most effective exchange system for diffusional transfer.
Figure 9a was prepared following immersion fixation(i.e. fresh, excised tissue samples measuring 2x1x1mm were immersed in the fixative for two hours), which caused severe collapse of fetal capillaries and maternal blood lacunas.
Figure 9b was prepared following supra-vital perfusion fixation of the maternal vascular system (i.e. the fixative was instilled into the uterine and ovarian arteries whereas mother animal and fetuses were in barbiturate anesthesia). Note that both, fetal (containing red blood cells) and maternal (devoid of blood) vessel lumina are wider than following immersion fixation, however, the maternal vessels smaller as compared to the fetal vessels.
Figure 9c was prepared following supra-vital perfusion fixation of the fetal vascular system (i.e. instillation of the fixative into the umbilical arteries whereas mother animal and fetuses were in barbiturate anesthesia). Note that both, fetal capillaries (devoid of blood) and maternal blood lacunae (containing erythrocytes) are much wider than following immersion fixation. However, different from the results of maternal perfusion fixation (Figure 9b), the width of the unperfused maternal blood lacunae considerably exceeds that of the perfused fetal capillaries.
Quantitative structural data:
When calculating the means following fetal and maternal perfusion fixation, quantitative analysis of the guinea pig placenta gives the following data:
Volume composition of the term guinea pig main placenta (interlobium and labyrinth):
- syncytiotrophoblast: 31.3%
- endothelium, other vascular wall cells, connective tissue: 13.7%
- fetal vascular lumina: 17.5%
- maternal lacunar lumina: 37,5%
In conclusion, 55% of the placental volume are vascular lumina filled with blood. This explains the sensitivity of this organ to postpartum vascular collapse, post-mortem changes, fixation artifacts, etc.
Mean materno-fetal diffusion distance at term: 3.2 µm
Surface data at term:
- maternal vascular luminal surface: 0.12 m2/cm3
- fetal vascular luminal surface: 0.11 m2/cm3
Most of these data (volume of syncytiotrophoblast, fetal vessel lumina, maternal blood volume, diffusion distance, ratio of fetal to maternal vascular surfaces) are surprisingly similar to those of the term human placenta. The only remarkable difference is the share of fetal connective tissue which, in the guinea pig, is close to zero whereas in the human placenta it amounts to > 20%.
Trophoblast turnover: The biology of syncytiotrophoblast in the human placenta, that is the mechanisms of its formation and regeneration as well as the extrusion of aged syncytiotrophoblast ('trophoblast turnover') have become well understood in the mean time (for review see Benirschke & Kaufmann, 2000): Villous cytotrophoblast provides a pool of proliferating stem cells which upon leaving the cell cycle start differentiation for about 2 days. Thereafter, syncytial fusion takes place. Upon syncytial fusion, the syncytial nuclei loose their generative potential: DNA replication is completely stopped; transcription of RNA is downregulated to unmeasurable values (Huppertz et al., 1999). Therefore, not only for its growth but also for its own survival and to fulfill the numerous transport and synthetic functions, syncytiotrophoblast depends on continuous input of freshly transcribed mRNA and freshly translated proteins. Without syncytial inclusion of new cytotrophoblast, syncytiotrophoblast dies within two to three days. The fusion rate required for survival by factor 6 exceeds the needs for syncytial growth. The resulting excess quantities of 'aged' nuclei are extruded by apoptotic mechanisms into the maternal blood. The average survival time of a nucleus in the syncytiotrophoblast was calculated to be around 20 to 30 days (Benirschke & Kaufmann, 2000).
Guinea pig syncytiotrophoblast looks like human villous syncytiotrophoblast structurally and the mode of syncytial fusion (Firth et al., 1980) seems to be the same as described for the human (for review see Benirschke & Kaufmann, 2000). Therefore, it is surprising to note, that in the second half of the guinea pig pregnancy cytotrophoblast is largely missing in the placental labyrinth. Syncytial fusion has only been observed throughout the first weeks of guinea pig gestation. This raises the question as to the nature of syncytiotrophoblast in the guinea pig, whether, different from the human, it does not depend on continuous syncytial fusion for growth and its own regeneration; whether syncytiotrophoblast nuclei in the guinea pig perhaps has maintained a certain degree of nucleic acid metabolism; whether syncytiotrophoblast in the guinea pig is really a true syncytium that is exclusively derived by fusion of former cellular trophoblast, or whether it has perhaps a plasmodial character (formation of multinuclear structures by nuclear division without subsequent cellular division); whether syncytiotrophoblast in the human and in the guinea pig are really as analogous structures, as is generally assumed. Unfortunately, up to now respective studies of trophoblast turnover in the guinea pig are completely missing.
6) Umbilical Cord and Larger Fetal Vessels
Two umbilical arteries enter the main placenta via the umbilical cord (Figure 10). On reaching the main placenta, they branch into 4 to 6 big 'chorionic' arteries lying superficially under the amnionic cover. A further branch vertically traverses the main placenta and supplies the subplacenta via the central excavation. The 'chorionic' arteries repeatedly branch dichotomously, always one of the arteriolar daughter branches penetrating the interlobium and following the surface of the labyrinthine lobules. From these arterioles, the labyrinthine capillaries originate. The capillaries fuse in the lobular centers to form one central lobular venule which, upon fusion with the other lobular venules and the subplacental vein, finally form the umbilical vein. Starck (1957) described the cord of the guinea pig as being 2.5 cm long.
Umbilical vessels in the guinea pig are not easy to handle experimentally since they tend to contract irreversibly and completely following even the slightest mechanical irritation. Prior to cannulation of the umbilical arteries, careful paravascular infiltration of 4% formaldehyde solution into the umbilical mesenchyme proved to be useful since it reduced the tactile sensitivity of the vessels.
The maternal blood supply of the guinea pig uterus and placenta are summarized in figures 10 to 12.
Maternal arterial blood to the placenta is jointly supplied by the ovarian and the uterine arteries, which on either side form a long anastomosis, the arcade artery which is located at the base of the mesometrium (Fig. 11). This arcade artery gives rise to numerous mesometrial (radial) arteries, some of which end as myometrial arteries, supplying non-pregnant uterine segments, others transforming into uteroplacental arteries and supplying the implantation sites.
As a specialty of caviomorphs, pregnancy-induced dilatation of uteroplacental arteries extends far beyond the uterine wall deeply into the mesometrium (see. Figs 13 and 14). As will be described in detail in section 9, this process is induced by trophoblast invasion. And when the response to prostaglandin and other mediators of different arterial beds was studied, it was found that the vessels less infiltrated by trophoblast were more responsive (Clausen et al., 2003).
Upon passing the uterine wall, 3 to 4 uteroplacental arteries give off smaller branches to the subplacenta and then enter the main placenta. For the last centimeter before reaching the main placenta already, considerable parts of the arterial wall are infiltrated and partly replaced by invasive trophoblast. Upon reaching the interlobium of the main placenta, the remaining maternal vascular tissue elements are replaced by trophoblast and the arteries are transformed into rigid mere trophoblastic tubes. These pass the interlobium and enter the labyrinthine lobes, where they finally branch into numerous arteriolar blood lacunae that are located in the centers of the labyrinthine lobes. Here, the latter give rise to capillary lacunae which centrifugally pass the labyrinth and supply it with maternal blood (see section 5).
Drainage of venous blood starts at the surface of the labyrinthine lobes. Here, the maternal capillary blood lacunae of the interlobium collect the venous blood in a web-like channel system which embeds all labyrinthine lobes. The venous blood of the interlobium flowing back maternally is collected in a large venous ring that surrounds the transition of the main placenta to the subplacenta (Fig. 12 b, c). This venous ring is partially embedded into the interlobium (where it has a trophoblastic wall) and partially surrounded by endometrial tissues, where its wall is composed of endothelium and medial smooth muscle cells.
This basal venous ring is drained by 3 to 4 uteroplacental veins (Fig. 12 a). These accompany the respective uteroplacental arteries. Additional drainage comes via mesometrial veins and an arcade vein that are finally connected to the ovarian and uterine veins.
8)
Extraplacental Membranes
In all rodents, the yolk sac placenta is the second most important maternal/fetal
transport organ besides the chorioallantoic placenta. In the guinea pig
as in many other rodent species, it was found to play a particular role
in maternal-to-fetal protein transfer (e.g. immunoglobulins) (Enders &
King 1970; King 1972). This is further supported by the fine structural
studies of King (1982) and King & Enders (1970).
The yolk sac placenta has its own fetal vascular supply that is provided
by vitelline vessels, derived from the upper mesenteric artery and vein
that leave the fetal abdominal cavity via the umbilicus. They accompany
the umbilical vessels in the umbilical cord for about half of its length
until they branch off (Fig. 10). There is no yolk sac-specific maternal
vascular supply, but nutrients and metabolites from the maternal circulation
reach the yolk sac via diffusion from the maternal endometrial stroma,
closely attached to the yolk sac.
Vitelline transport routes: Consequently, to reach the fetal circulation,
maternal immunoglobulins have to pass the following tissue layers:
the maternal endometrial capillary endothelium (paracellularly);
the endometrial stroma (by extracellular lymphatic flow);
the endometrial epithelium (mostly paracellularly);
the yolk sac epithelium (partly paracellularly, partly by receptor-mediated
vesicular transport);
the vitelline connective tissue (by interstitial fluid flow and by diffusion);
the vitelline capillary endothelium (partly paracellularly, partly be
vesicular shuttle).
Despite of this long and seemingly complicated route, this appears to
be an effective transfer route for macromolecules according to the data
of King & Enders (1970) and King (1972).
Structural organization: The yolk sac placenta can be subdivided into
the following sections with respect to structure and location (Figures
1, 2, 13):
The visceral yolk sac, opposed to the uterine epithelium (for its structure
see Figure 6): This appears to be the most effective maternal/fetal exchange
area within the yolk sac. Being close to the main placenta (Figure 2),
this part of the yolk sac placenta is characterized by a highly columnar
yolk sac epithelium that is ultrastructurally equipped with all the organelles
required for effective macromolecular transport.
The visceral yolk sac folds insert at the fetal surface of the main placenta.
As can be seen from Figures 1 and 2, these yolk sac folds are no longer
opposed to the endometrial surface but rather to the surface of the chorioallantoic
placenta. Structurally, they are similar to the section described above.
In the absence of a direct endometrial source of maternal proteins, this
section can only take up proteins from the yolk sac lumen (surrounded
by visceral yolk sac, parietal yolk sac and endometrial surface).
The parietal yolk sac (Figure 2): This is a layer of yolk sac epithelium
which is directly connected to the lateral surfaces of the main placenta
by a kind of basement membrane (Reichert's membrane). The functional purpose
of the parietal yolk sac is still obscure. This section of the yolk sac
has no fetal vascular supply and it can therefore, not contribute to the
maternal/fetal exchange. Also, experimental data by King (1972) revealed
considerably reduced uptake of tracer proteins when compared with that
of the visceral sections of the yolk sac. It more likely acts as a kind
of mechanically important surface epithelium of the main placenta.
9) Trophoblast External to Barrier
Caviomorph rodents are examples among mammals of the most intense trophoblast
invasion. This invasion is not restricted to the uterus but it goes far
beyond the uterine walls, deeply into the peritoneal cavity. In spite
of this excessive invasion, the process is still only poorly studied in
the guinea pig and other caviomorphs. The invasion appears to start at
and around the subplacenta, another structure specific to caviomorph rodents.
Subplacenta: The subplacenta is formed as the basal part of the chorioallantoic
placenta at about day 14. Because of its most basal position, it is the
first part of the chorioallantoic placenta supplied by maternal blood
channels, and is the latest to be reached by fetal vessels. In fact, in
the maternal blood lacunae of the future subplacenta maternal blood flow
ceases as early as between days 20 and 27 post conception by formation
of blood clot, while establishment of a fetal circulatory system in this
area is starts at day 23 and is completed only at day 32 (Uhlendorf &
Kaufmann, 1979). Consequently, because of its extreme position, this particular
basal tip of the early cone-shaped chorioallantoic placenta (Fig. 3a),
is never simultaneously supplied by the maternal and
the fetal circulation; therefore, it never participates in fetal/maternal
exchange.
Structurally it consists of highly folded layers (Figs. 2; 3a,b; 10) of
fetal connective tissue (roof of the central excavation) (Fig. 3a, 10);
basally followed by one or more layers of cytotrophoblast, the only cytotrophoblast
surviving in the chorioallantoic placenta to the second half of gestation
(Fig. 10); at its endometrial surface, the cytotrophoblast is covered
by a thick, bizarre-shaped layer of syncytiotrophoblast (Figs. 2, 13);
the meshwork of maternal blood lacunae of the latter is perfused by maternal
blood only until the end of the third week; thereafter blood flow stops
by blood clot and, with the latter organization, the lacunae gradually
disappear. From the basal tips of syncytiotrophoblastic folds, root-like
syncytial extensions (syncytial streamers) deeply penetrate the endometrium.
At about day 52 degenerative processes start in the subplacental syncytiotrophoblast
and later include also cytotrophoblast and connective tissue. On day 60
degeneration of the subplacenta is completed. As a consequence of these
processes, the main placenta is connected to the junctional zone only
by 3 to 4 bundles of uteroplacental vessels from this date onwards; these
are arranged around the large degenerative zone of the former subplacenta.
After delivery of the fetus, separation of the placenta occurs across
this degenerative zone, so that only the uteroplacental vascular bundles
need to be disconnected. Therefore, the resulting postpartum wound area
is very small. This fact may support the next conception of guinea pigs
as early as within 24 hours after delivery during the so-called postpartum
estrus.
The functional importance of the subplacenta is still a mystery. The subplacenta
does not serve as a fetal/maternal exchange organ in any stage of pregnancy.
By degeneration a few days prior to delivery, it contributes to minimizing
the uterine wound area postpartum.
Throughout pregnancy the subplacental syncytiotrophoblast (guinea pig:
Kaufmann & Davidoff, 1977; Wolfer & Kaufmann, 1980; chinchilla:
King & Tibbitts, 1976) produces large amounts of secretory granules
with glycoprotein character which are secreted into the maternal blood
lacunae. Because of the absent maternal circulation in the lacunae, the
unknown secretory product is accumulated within the subplacenta and set
free only during its degeneration. It was speculated that this secretion
assists either in separation of the placenta or in postpartum removal
of cellular debris and wound healing (Wolfer & Kaufmann, 1980).
As will be described in the following paragraphs, throughout pregnancy
the subplacenta is the main, if not the exclusive source of trophoblast
invasion. Therefore it may act as the guinea pig equivalent of the cell
columns in the villous placentas of primate. Unfortunately, this aspect
has not yet been studied in detail since the biology of human cell columns
and their role in trophoblast invasion were still largely unknown when
most studies on subplacenta and main placenta of the guinea pig were performed.
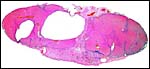
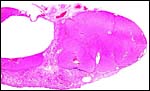
Interstitial trophoblast invasion: The histological survey picture in
Figure 14 illustrates the intensity of trophoblast invasion emerging from
the subplacental area. As shown in Figures 2, 3b, 10 and 15, this is the
only zone of contact between chorioallantoic placenta and endometrium,
and consequently the only potential source for trophoblast invasion. Our
own recent re-evaluation of old slides of subplacenta and junctional zone
of the guinea pig, the chinchilla, the capybara and the degu suggests
the following types and routes of trophoblast invasion in this area (Figure
13):
Invasion of syncytial roots (so-called syncytial streamers) occurs from
the subplacenta close toward the myometrium. This process starts concomitant
with implantation of the blastocyst. Respective syncytial streamers can
be found in the junctional zone until term.
Interstitial invasion of cytotrophoblast begins in the third week of gestation.
Its existence requires that the syncytiotrophoblastic shell, separating
all cellular components of the chorioallantoic placenta from the endometrium,
represented by the syncytial layer of the subplacenta (see above), becomes
locally interrupted. This is found most often in the neighborhood of uteroplacental
vessels passing the subplacenta and entering the basal surface of the
main placenta. At such syncytial discontinuities, plugs of cytotrophoblast
protrude into the endometrium. Their cellular phenotype shows certain
similarities with cell columns in the human. However, their trophoblast
cells as well as the daughter cells that penetrate deeper into the endometrium/junctional
have never been studied for molecular signs of invasiveness, to the best
of our knowledge.
Abdominal invasion of cytotrophoblast: This is a special feature of caviomorph
pregnancies. Trophoblastic cells leave the subplacenta and penetrate the
full thickness of the uterine wall, reach the perimetrial surface of the
uterine wall and the peritoneal surface of the mesometrium. In this location,
invasive trophoblast adopts a mesothelial phenotype and replaces large
parts of the original mesothelium as a monolayer of highly flattened cells
(Nanaev et al., 1995). Microscopically, the peritoneal cover provided
by trophoblast cells can be identified by immunohistochemistry. Macroscopically,
it can be identified by naked eye upon opening the peritoneal cavity,
since the peritoneal trophoblast surface is less shiny than the original
mesothelial surface.
Interestingly, these cells secrete nitric oxide (Nanaev et al., 1995)
and thus have a vasodilatory effect on the mesometrial and uteroplacental
arteries wherever the trophoblast cells replace the original mesothelium
in the surrounding of these arteries (Figs. 13 and 14).
Endovascular trophoblast invasion in the guinea pig (Nanaev et al., 1995)
is structurally very similar to endovascular trophoblast invasion in the
human (for review see Benirschke & Kaufmann, 2000). As in the case
for the human placentation, the point of entry is not yet certain. We
suggest that the trophoblast cells pass the arterial walls and enter the
arterial lumens where the arteries pass the subplacenta and enter the
placental parenchyma (Fig. 14). This is very likely so because (a) of
structural findings and (b) since the subplacenta in most stages of gestation
is the only potent source of cytotrophoblast. From the point of entry,
the trophoblast cells migrate proximally against the blood flow, replacing
the endothelium and also replacing parts of the surrounding arterial wall
(Nanaev et al., 1995). Whether the mural infiltration is supported by
invasive interstitial trophoblast ('intravasation') or depends only on
endovascular trophoblast cells ('extravasation') remains to be clarified.
It is interesting to note that, different from the classical views, endovascular
trophoblast invasion is peripherally always less expansive than is arterial
dilatation. Endovascular invasion of the already maximally dilated arteries
seems to be a consequence rather than the reason for dilatation.
Pregnancy-induced modification of uteroplacental arteries and their reconstitution
following delivery: Mesometrial and uteroplacental arteries undergo a
series of dramatic structural and functional changes during and after
pregnancy (Nanaev et al., 1995; 2000):
The mesometrial arteries and their uteroplacental continuations into the
pregnant segments of the uterine wall are slender tubes (luminal diameters
< 0.3mm) during the first two weeks post conception.
As early as in the third week, first invasive trophoblast cells can be
found lining the peritoneal surface attached to the uteroplacental arteries.
The corresponding arterial segments dilate maximally and reach luminal
diameters of 1.5 to 2.0 mm. In spite of their maximal dilatation, structural
changes of the arterial walls cannot be observed at this stage. With advancing
gestation, peritoneal trophoblast invasion into the mesometrium continues
and is accompanied by the progression of arterial dilatation in proximal
directions (towards the arcade artery).
At this stage, trophoblastic infiltration of the already maximally dilated
arterial wall can be observed only close to the placenta: The trophoblast
cells largely replace the endothelium, basal laminas and all elastic elements.
Also, smooth muscle media cells appear to disappear. But, as was shown
by Nanaev et al. (1995) in the guinea pig, the loss of smooth muscle media
cells is caused by dedifferentiation to undifferentiated myoblasts, rather
than by destruction. Due to these processes, the arteries are transformed
into rigid, inelastic tubes that no longer are responsive to maternal
vasoregulation. Only about the distal (placental) half of the mesometrial
arteries becomes transformed in this way; the proximal halves are not
reached by trophoblast invasion and remain undilated.
Following delivery, the invasive trophoblast cells die and disappear within
about five days thereafter. In analogy to human trophoblast invasion (for
review see Kaufmann et al., 2003), it can be assumed that trophoblast
cells enter the invasive pathway only after leaving the cell cycle; moreover,
their post-mitotic, invasive life-span is limited to a few days and thereafter
terminated by apoptosis. Therefore, invasive trophoblast unavoidably disappears
within a few days after delivery, since the apoptotic cells can no longer
be replaced by new generations of post-mitotic daughter cells derived
from the placenta. In parallel with the disappearance of the invasive
trophoblast cells, arterial myoblasts start re-differentiation into mature
and fully active smooth muscle media cells. The luminal lining of the
arteries is reconstituted by proliferation of the remaining endothelial
cells which replace the dying endovascular trophoblast cells. Eight days
after delivery, the mesometrial arteries are fully reconstituted and look
like normal arteries.
These events allow quick and complete adaptation of the uterine arterial
blood supply to pregnancy conditions in the guinea pig, and to a complete
reversal within a few days after delivery. It is still unknown whether
the data can be transferred to the human condition. Observations by Catherine
Craven point in this direction. This author found in the human that arterial
dilation starts prior to infiltration of the arteries by trophoblast (Craven
et al., 1998). Moreover, the same authors showed that even after trophoblastic
infiltration of the arteries and apparent loss of smooth muscle media
cells, alpha-smooth muscle actin could still be detected in the vessel
walls. This suggests that the muscle was not destroyed but rather only
de-differentiated is also true in the human (Craven & Ward, 1996;
for review see Kaufmann et al., 2003).
The endometrium shows moderate decidualization where it is invaded by trophoblast. In a detailed electronmicroscopic study of decidua during the entire course of pregnancy, Wynn (1965) found remarkable metabolic activity and large numbers of microvilli.
11) Various Features
A special feature of all caviomorph placentas is the subplacenta. Since
it is the source of invasive trophoblast, it has been described in that
context (see section 9: Trophoblast External to Barrier).
12) Endocrinology
Endocrine control of pregnancies in the guinea pig shows some homologies
with that of human pregnancy, but there are also some remarkable differences:
Similar to the human, the endocrine activity of the corpus luteum is taken
over by the placenta during the course of pregnancy. Lutectomy in the
middle and in the second half of guinea pig gestation are not followed
by abortion. Also, caviomorph rodent placentas secrete a protein with
progesterone-binding capacity (PBP). According to Perrot-Aplanat &
David-Ferreira (1982) PBP is synthesized within the syncytiotrophoblast
of the interlobium and secreted into the maternal circulation. PBP increases
the biological half-life of progesterone. Also similar to the human and
to the tupaia, parturition in the guinea pig is not induced by a fall
of progesterone levels. Rather, progesterone levels cease only after parturition
due to absence of their secretory source, the placenta.
On the other hand, to our best knowledge, a guinea pig analogue to the
human chorion gonadotropin has not yet been identified.
13) Genetics
Guinea pigs have 64 chromosomes (Hsu & Benirschke, 1968). Short arm deletions have been reported in the past. Hybrids of domestic guinea pigs with C. aperea, C. cutleri and C. fulgida are reportedly fertile although they are not commonly produced (Gray1972).
| Figure 16: Karyotypes of male and female guinea pigs. | |
Consideration of the extensive studies on immune phenomena in guinea pigs is beyond the scope of this chapter.
15)
Pathological Features
Due to the clearly defined labyrinthine system of maternal blood lacunae,
the guinea pig placenta has been used as a model to induce placental infarction:
Systemic treatment with inhibitors of glycolysis (from hours to days),
caused severe focal to generalized edematous changes in the syncytiotrophoblast
of the interlobium and thereby blockage of venous drainage of maternal
blood. Depending on dose and length of treatment, smaller labyrinthine
red infarcts or complete degeneration of the placenta were induced (Kaufmann,
1975). In spite of phenotypic similarities with certain infarctions in
the human placenta, it remains speculative whether the data obtained with
this model can be transferred to the human condition.
Guinea pigs may suffer a variety of skin conditions, intestinal disorders
and other diseases that are easily accessible through web sites such as,
http://www.oginet.com/pgurney/.
16) Physiological Data
Arterial blood pressure in the distal part of mesometrial (radial) arteries
(Moll & Kuenzel, 1973) is:
- in the non-pregnant state: 44 +/- 7 mm Hg;
- in pregnancy at term: 12 +/- 3 mm Hg.
Age
in days |
Main
placenta(ml/min) |
Junctional
zone including subplacenta (ml/min) |
Pregnant
uterus as a whole (ml/min * g) |
22
|
0.4 |
||
25
|
0.6 |
0.06 |
0.475 |
30 |
1.1 |
0.12
|
0.385 |
35
|
2.2
|
0.19
|
0.320 |
40
|
3.6 |
0.22
|
0.260 |
45
|
6.0 |
0.20 |
0.210 |
50
|
6.3 |
0.14 |
0.165 |
55
|
7.0
|
0.09
|
0.130 |
60 |
7.3 |
0.08 |
0.100 |
65 |
7.5 |
0.08
|
0.080 |
17) Other Resources
When one attempts to time the mating of guinea pigs, this results in rather low pregnancy rates. Pregnancy yield can be much improved by keeping the guinea pigs in small, permanently polygamous breeding groups (1 male per 4 to 6 females). Under these conditions, 75% of females conceive within 24 hours following their last delivery, i.e. within the so-called post-partum estrus. The term of conception is then, cum grano salis, identical to the first day after delivery.
When one breeds in this fashion, it becomes important to know whether a sow became pregnant, and how the new pregnancy develops since abortions and periods of halted or retarded fetal development are quite common, as in deer.
A simple method of palpation allows identification of pregnancy from day 10 to 15 onwards, and allows accuracy of timing within +/- 3 days. For the examination of the left uterine horn, the female is immobilized by the right hand as illustrated in Figure 17a. The thumb of the left hand is pressed again the mother's spine, the four other finger tips slightly press the abdominal wall against the thumb. When drawing the hand laterally, the uterus should be felt between thumb and finger tips sooner or later. In the case of pregnancy, swellings of the uterine horn can be detected. The same is repeated with the right hand for the right uterine horn. Depending on size and quality of the palpational findings, at least six pregnancy stages can be identified (Figure 17 b):
Stage 1 (days 10 to 15). Firm, pea-sized swellings from about 5 to 6 mm diameter.
Stage 2 (days 15 to 25). Firm, hazel nut-sized swelling, about 10 to 15 mm in diameter.
Stage 3 (days 25 to 35). Elastic, slightly oval bodies, 15 to 30 mm in diameter.
Stage 4 (days 35 to 45): Cylinder-shaped, heterogeneous bodies, 3.5 to 5 cm long, containing one to two solid parts (head and pelvis).
Stage 5 (days 45 to 55): Length of the cylindrical fetus 5 to 7 cm. Head, thorax with few ribs and pelvis can be identified.
Stage 6 (days 55 to term): Length of the fetus 7 to 10 cm. The hard, knobby head is about 2.5 cm long, orbits and mandible can be identified by palpation.
This method allows a sufficiently safe estimation of pregnancy age of +/- 5 days and, with some experience, accuracy of +/-2 to 3 days can be reached.
Time of approaching term can be identified by palpating the symphysis. The pubic bones start to separate 3 to 4 days before term and stand apart 1 to 2 cm at term.
The guinea pig placenta has been studied and the data have been interpreted mainly in the 1960s and 1970s. In that period, not too much was known about the biology of the human trophoblast (such as turnover of villous trophoblast, trophoblast apoptosis, and trophoblast invasion) as well as angiogenesis. Re-evaluation of certain aspects of the biology of the guinea pig placenta in the light of these new insights into human placental biology may therefore very likely further or modify our understanding of this organ.
Acknowledgement
I am most grateful to my anatomy teacher, Theodor Heinrich Schiebler,
who in the 1960s probably was the first to realize the potential of the
guinea pig as experimental model and who encouraged his pupils and colleagues
[Müller, Vollrath, Davidoff, Kaufmann and others] to provide a detailed
structural analysis of this organ.
The actual placental slides in H&E were kindly supplied by Dr. Brian Stacey of the San Diego Zoo's department of pathology.
References
[Except when other citations were given, the data presented above are
from Kaufmann & Davidoff (1977).]
Benirschke, K. and Kaufmann, P.: The Pathology of the Human Placenta. 4th ed. Springer-Verlag, NY, 2000.
Bjellin, L., Sjoequist, P. and Carter, A.: Uterine, maternal placental and ovarian blood flow throughout pregnancy in the guinea-pig. Z. Geburtsh. Perinat. 179:179-187, 1975.
Clausen, H.V., Larsen, L.G. and Carter, A.M.: Vascular reactivity of the preplacental vasculature in guinea pigs. Placenta 24:686-697, 2003.
Craven, C.M., Morgan, T. and Ward, K.: Decidual spiral artery remodeling begins before cellular interaction with cytotrophoblasts. Placenta 19:241-252, 1998
Craven, C.M. and Ward, T.: Alpha-smooth muscle actin is preserved in arteries showing physiologic change. Placenta 17:A17, 1996.
Duval, M.: Le placenta des rongeures. III. le placenta du cochon d'Inde. J. Anat. (Paris) 28:58-408, 1892.
Ely, P.A.: The placental transfer of hexoses and polyols in the guinea-pig, as shown by umbilical perfusion of the placenta. J. Physiol. 184:255-271, 1966.
Faber, J. and Hart, F.M.: The rabbit placenta as an organ of diffusional exchange. Comparison with other species by dimensional analysis. Circulat. Res. 19:816-833, 1966.
Firth, J.A., Farr, A. and Bauman, K.F.: The role of gap junctions in trophoblastic cell fusion in the guinea-pig placenta. Cell Tissue Research 205:311-318, 1980.
Garris, D.R.: Intrauterine growth of the guinea pig fetal-placental unit throughout pregnancy: regulation by utero-placental blood flow. Teratology 29:93-99, 1984.
Gotch, A.F.: Mammals - Their Latin Names Explained. Blandford Press, Poole, Dorset, 1979.
Gray, A.P.: Mammalian Hybrids. A Check-list
with Bibliography. 2nd edition.
Commonwealth Agricultural Bureaux Farnham Royal, Slough, England, 1972.
Hsu, T.C. and Benirschke, K.: An Atlas of Mammalian Chromosomes. Springer-Verlag, NY. Volume 2, Folio73, 1968.
Huppertz, B., Frank, H.G., Reister, F., Kingdom, J., Korr, H. and Kaufmann, P.: Apoptosis cascade progresses during turnover of human trophoblast: analysis of villous cytotrophoblast and syncytial fragments in vitro. Lab. Invest .79:1687-1702, 1999.
Kaufmann, P.: Experiments on infarct genesis caused by blockage of carbohydrate metabolism in guinea pig placentae. Virchows Arch. A, Pathol. Anat. Histopathol. 368:11-21, 1975.
Kaufmann, P., Black, S. and Huppertz, B.: Endovascular trophoblast invasion. Biol. Reprod. (in press), 2003.
Kaufmann, P. and Davidoff, M.: The guinea-pig placenta. Adv. Anat. Embryol. Cell Biol. 53: 1-91, 1977.
King, B.F.: The permeability of the guinea pig parietal yolk sac placenta to peroxidase and ferritin. Amer. J. Anat. 134:365-376, 1972.
King, B.F. and Enders, A.C.: The fine structure of the guinea pig visceral yolk sac placenta. Amer. J. Anat. 127:397-414, 1970.
King, B.F.: A freeze-fracture study of the guinea pig yolk sac epithelium. Anat. Rec. 202:221-230, 1982.
King, B.F. and Enders, A.: Protein absorption and transport by the guinea pig visceral yolk sac placenta. Amer. J. Anat. 129:261-288, 1970.
King, B.F. and Tibbitts, F.D.: The fine
structure of the chinchilla placenta. Amer. J. Anat. 145:33-56, 1976.
Miglino, M.A., Carter, A.M., Ambrosio, C.E., Bonatelli, M., de Oliveira, M.F., dos Santos, Ferraz, R.H., Rodrigues, R.F. and Santos, T.C.: Vascular organization of the hystricomorph placenta: a comparative study in the agouti, capybara, guinea pig, paca and rock cavy. Placenta 25:438-448, 2004.
Moll, W. and Kuenzel, W.: The blood pressure in arteries entering the placentae of guinea pigs, rats, rabbits and sheep. Pfluegers Arch. Ges. Physiol. 338:125-131, 1973.
Nanaev, A., Chwalisz, K., Frank, H.G., Kohnen, G., Hegele-Hartung, C., Kaufmann, P.: Physiological dilation of uteroplacental arteries in the guinea pig depends on nitric oxide synthase activity of extravillous trophoblast. Cell Tissue Research 282:407-421, 1995.
Nanaev, A.K., Kosanke, G., Reister, F., Kemp, B., Frank, H.G., Kaufmann, P.: Pregnancy-induced de-differentiation of media smooth muscle cells in uteroplacental arteries of the guinea pig is reversible after delivery. Placenta 21:306-312, 2000.
Perrot Applanat, M. and David Ferreira, J.F.: Immunocytochemical localization of progesterone-binding protein (PBP) in guinea-pig placental tissue. Cell Tissue Research 223:627-639, 1982.
Rowell, L.C., Keyes, L.E. and Moore, L.G.: Chronic hypoxia diminishes pregnancy-associated DNA synthesis in guinea pig uteroplacental arteries. Placenta 21:313-319, 2000.
Starck, D.: Ueber die Länge der Nabelschnur bei Säugetieren. Z. Säugetierk. 22:77-86, 1957.
Uhlendorf, B. and Kaufmann, P.: Die Entwicklung des Plazentastieles beim Meerschweinchen. Anat. Histol. Embryol. 8:233-237, 1979.
Webster, S.H. and Liljegren, E.J.: Organ : Body weight ratios for certain organs of laboratory animals. II. The guinea pig. Amer. J. Anat. 85:199-230, 1949.
Weir, B.J.: Reproductive characteristics of hystricomorph rodents. Symp. Zool. Soc. London 34:265-301, 1974.
Wolfer, J. and Kaufmann, P.: Die Ultrastruktur der Merrschweinchen-Subplacenta. Anat. Histol. Embryol. 9:29-43, 1980.
Wynn,
R.M.: Electron microscopy of developing decidua. Fertil. Steril. 16:16-26,
1965.