|
(Clicking
on the thumbnail images below will launch a new window and a larger
version of the thumbnail.)
|
Connochaetes taurinus; C. gnou
Order: Artiodactyla
Family: Bovidae
1) General Zoological Data
Two species are recognized: the much more common brindled gnu (also white-bearded or blue gnu) and the smaller white-tailed gnu (black gnu). Connochaetes derives from konnos (Greek = beard) and khaite (flowing hair), while gnou is said to be Hottentot for the antelope (Gotch, 1979). The white-tailed gnu is now very rare and perhaps limited to areas of Namibia, while the blue wildebeest exists in larger regions of South and Southeast Africa and is well-known because of its large migrations. It is also much more common in zoological gardens, the former being very uncommonly seen now. Several subspecies have been described of the blue gnu that are mainly regional color variants. Adults weigh up to 275 kg, neonates weigh around 22 kg. Longevity is over 21 years (Jones, 1993).
Corbet & Robinson (1991) estimated the divergence time of these two species as 1 MYA from mtDNA studies. Arctander et al. (1999) studied mtDNA regions of three alcelaphine species (gnu, topi, hartebeest) and found them to be closely related. Moreover, they provided evidence that they had been much more widely distributed over Africa in the past.
 |
Connochaetes gnou. |
 |
Connochaetes taurinus taurinus. |
 |
Connochaetes taurinus albijubatus. |
Reproduction of wildebeest is highly seasonal in Africa. The length of gestation is 8-9 months (250-260 days) with single offspring weighing 20-22 kg. Age of first conception is about 28 months (Mentis, 1972). Placentas weigh between 1,500 and 1,800 g. Estes & Estes (1979) reviewed the social organization of wildebeest and make reference to the high neonatal survival among wildebeest calves in the Ngorongoro Park.
3)
Implantation
No details are known of early implantations or interaction of blastocyst
with endometrium.
4)
General Characterization of the Placenta
This is a polycotyledonary placenta; no perceptible difference was found
between the two species. Both have about 40 cotyledons, arranged in four
rows. The weight of one of my term brindled gnu placentas was 1,575 g
(37 x 11 cm). The placenta of a white-tailed gnu weighed 1,870 g. The
cotyledons measured up to 10 cm in greatest diameter and were remarkably
thin (0.3 cm). Hradecky (1983) published different numbers of cotyledons:
in both species there were four rows of cotyledons but the number of cotyledons
varied from 58 to 104, with a maximal size 11 x 6 cm. He found, roughly,
that one-half of the cotyledons were fewer than 5 cm in diameter. Later,
Hradecky (1988) described in more detail the structure of placentomes.
He measured the villi as being 8 mm long and indistinct at their tips.
They had few branches.
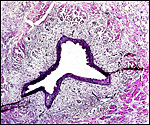
 |
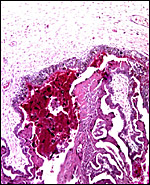
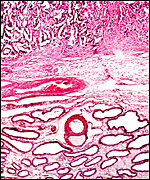
Implanted term placental cotyledon; uterus below. |
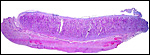
 |
Implanted term placental cotyledon; uterus below. |
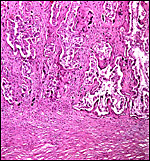
The barrier is epithelio-chorial and there is no infiltration of the uterus by trophoblast. Beneath the chorionic surface of at least one of the two mature placentas there was degeneration of the subchorionic tips of the maternal septa with some hemorrhage. A few of the cylindrical trophoblast beneath the chorion has incorporated yellow pigment. It was iron-staining negative. The villi are covered by a single layer of cuboidal trophoblast with a large number of binucleate cells, investigated in other ruminants by Wooding (1982) as well as by Atkinson et al. (1993). The latter produce glycoproteins and, most likely as in other ungulates, are responsible for the secretion of placental lactogen.
The umbilical cord contains 4 large blood vessels, an allantoic duct and a number of small blood vessels. Numerous foci of squamous metaplasia are found on the surface. Hradecky (1983) stated that the cord of these animals measures usually less than 20 cm.
No details have been published.
8)
Extraplacental membranes
Hradecky (1983) found small hippomanes in gnus; I have not found these
allantoic residues in my specimens.
9)
Trophoblast external to barrier
There is no trophoblastic infiltration of the uterus or endometrium.
10)
Endometrium
The uterus is bicornuate with a single cervix but deep division by the
uterine septum (Hradecky, 1982). There are four distinct rows of caruncles,
best seen in neonatal uteri. Here they are moderately edematous and lack
glands. The septa between villi are irregular and lined by a "syncytial
epithelium" (Hradecky, 1988).
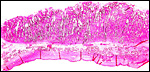
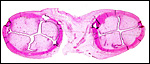 |
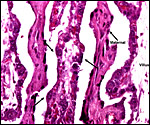
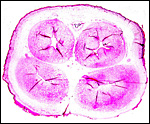
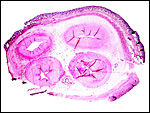
Two cross sections through a neonatal uterus with four pale caruncles each. |
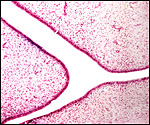 |
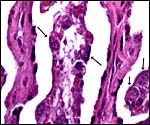
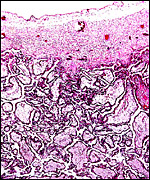
Higher magnification of endometrial caruncles in neonate. |
There are no special features worth noting. There is no subplacenta and there are no "metrial glands".
12)
Endocrinology
As in other ungulates, the placenta contains an abundance of binucleate
cells, thought to be responsible for placental lactogen production. The
origin and the multitude of lactogenic hormones have been discussed in
detail by Forsyth (1986). Neonatal testes had no interstitial cell stimulation.
The adrenal glands have conspicuously large medullary regions.
13)
Genetics
Both species have 58 chromosomes of identical shape, all acrocentrics
except for the largest chromosome, a submetacentric element (Wurster &
Benirschke, 1968; Hsu & Benirschke, 1971, 1974). Corbet & Robinson
(1991) also found no structural chromosomal differences between the two
species when employing more advanced banding techniques. They arrived
at a divergence time of 1 Million years from restriction fragment analysis
of mtDNA. They were astonished at the low heterogeneity in the population,
much like Grobler & v.d. Bank (1993). These authors studied the polymorphism
of 33 protein loci in three populations and determined that most polymorphism
existed in a population with 200 founders. They emphasized heterogeneity
as an important management tools for game ranches. Ralls et al. (1979)
had shown from captive populations that 85.7% of non-inbred offspring
survived until 6 months, while only 70.7% of inbreds did. Gray (1972)
reported on two putative hybrids between these species. One of these was
from Hagenbeck Zoo and reported in detail by Zukowsky (1967). The animal
came from a ranch in South Africa and had external features that betrayed
its hybrid status. It lived to 26-28 months and had cranial features that
were also intermediate between the two species. The other was at Zürich
Zoo and then went to Leipzig without further details available. Their
reproductive tracts were not reported.
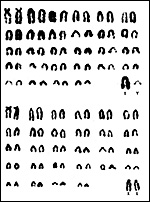 |
Karyotypes of white-tailed gnu, Connochaetes gnou. |
I am not aware of any immunological studies other than the search for antibodies to viruses.
15)
Pathological features
An outstanding consideration of gnu pathology is their carrier state for
the virus of malignant catarrhal fever (alcelaphine herpesvirus 1 - AHV1).
This infection has caused many problems in zoological parks (Metzler,
1991; Flach et al., 2002) and has been differentiated from the virus of
sheep association. Rossiter et al. (1983) attempted to identify the virus
from delivered placentas and suggested that it is rapidly inactivated
by sunlight. It is probable, however, that calving and the simultaneous
presence of the virus is related to infection of livestock at that time.
Neonatal diarrhea is sometimes due to rotavirus infection (Baumeister
et al., 1983). Anaplasma marginale was identified in gnus but apparently
has no pathologic sequelae (Kuttler, 1984). Boomker et al. (2000) enumerated
the parasites identified from a variety of South African ungulates. Peter
et al. (1998) infected animals with the agent causing heartwater disease
(Cowdria ruminantium) and established the importance of the tick
(Amblyomma hebraceum) in the endemic areas. In his experience with
numerous gnus at the San Diego Zoo, Griner (1983) found neonatal trauma
or malnutrition as principal causes of death, with rare infections. He
also noted that many blood vessels had irregular calcifications.
16)
Physiologic data
Watson (1976) reported on electroejaculation, semen characteristics and
preservation of semen. Basic hematologic values were provided by the study
of Peinado et al. (1999).
17)
Other resources
Numerous fibroblast cell lines of both species are available from the
"Frozen Zoo" at CRES at the San Diego Zoo by contacting Dr.
Oliver Ryder (oryder@ucsd.edu).
18)
Other remarks - What additional Information is needed?
Early stages of implantation are missing and there are no details on gestational
endocrine profiles.
Acknowledgement
The animal photographs in this chapter come from the Zoological Society
of San Diego.
References
Arctander, P., Johansen, C. and Coutellec-Vreto, M.A.: Phylogeography
of three closely related African bovids (tribe Alcelaphini). Mol. Biol.
Evol. 16:1724-1739, 1999.
Atkinson, Y.H., Gogolin-Ewens, K.J., Hounsell, E.F., Davies, M.J., Brandon, M.R. and Seamark, R.F.: Characterization of placentation-specific binucleate cell glycoproteins possessing a novel carbohydrate. J. Biol. Chem. 268:26679-26685, 1993.
Baumeister, B.M., Castro, A.E., McGuire-Rodgers, S.J. and Ramsay, E.C.: Detection and control of rotavirus infection in zoo animals. J. Amer. Vet. Med. Assoc. 183:1252-1254, 1983.
Boomker,
J., Horak, I.G., Watermeyer, R. and Booyse, D.G.: Parasites of South African
wildlife. XVI. Helminths of some antelope species from Eastern and Western
Cape provinces. Onderstepoort J. Vet. Res. 67:31-41, 2000.
Corbet, S.W. and Robinson, T.J.: Genetic divergence in South African wildebeest:
comparative cytogenetics and analysis of mitochondrial DNA. J. Hered.
82:447-452, 1991.
Estes, R.D. and Estes, R.K.: The birth and survival of wildebeest calves. Z. Tierpsychol. 50:45-95, 1979.
Flach, E.J. Reid, H., Pow, I. and Klemt, A.: Gamma herpesvirus carrier status of captive artiodactyls. Res. Vet. Sci. 73:93-99, 2002.
Forsyth, I.A.: Variation among species in the endocrine control of mammary growth and function: the roles of Prolactin, growth hormone, and placental lactogen. J. Dairy Sci. 69:886-903, 1986.
Gotch, A.F.: Mammals - Their Latin Names Explained. Blandford Press, Poole, Dorset, 1979.
Gray,
A.P.: Mammalian Hybrids. A Check-list with Bibliography. 2nd edition.
Commonwealth Agricultural Bureaux Farnham Royal, Slough, England, 1972.
Griner, L.A.: Pathology of Zoo Animals. Zoological Society of San Diego, San Diego, California, 1983.
Grobler, J.P. and van der Bank, F.H.: Genetic variability in South African blue wildebeest (Connochaetes taurinus). Comp. Biochem. Physiol. B. 106:755-762, 1993.
Hradecky, P.: Uterine morphology in some African antelopes. J. Zoo Anim. Med. 13:132-136, 1982.
Hradecky, P.: Placental morphology in African antelopes and giraffes. Theriogenology 20:725-734, 1983.
Hradecky, P., Mossman, H.W. and Stott, G.G.: Comparative histology of antelope placentomes. Theriogenology 29:693-729, 1988.
Hsu,
T.C. and Benirschke, K.: An Atlas of Mammalian Chromosomes. Springer-Verlag,
New York.Vol. 6, Folio 292, 1971; Vol. 8, Folio 393, 1974.
Jones, M.L.: Longevity of ungulates in captivity. Intern. Zoo Yearbk.
32:159-169, 1993.
Kuttler, K.L.: Anaplasma infections in wild and domestic ruminants: a review. J. Wildl. Dis. 20:12-20, 1984.
Matthee, C.A. and Robinson, T.J.: Cytochrome b phylogeny of the family bovidae: resolution within the alcelaphine, antilopini, neotragini, and tragelaphini. Mol. Phylogenet. Evol. 12:31-46, 1999.
Mentis, M.T.: A review of some life history features of the large herbivores of Africa. The Lammergeyer 16:1-89, 1972.
Metzler, A.E.: The malignant catarrhal fever complex. Comp. Immunol. Microbiol. Infect. Dis. 14:107-124, 1991.
Peinado, V.I., Celdran, J.F. and Palomeque, J.: Basic hematological values in some wild ruminants in captivity. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 124:199-203, 1999.
Peter, T.F., Anderson, E.C., Burridge, M.J. and Mahan, S.M.: Demonstration of a carrier state for Cowdria ruminantium in wild ruminants from Africa. J. Wildl. Dis. 34:567-575, 1998.
Ralls, K., Brugger, K. and Ballou, J.: Inbreeding and juvenile mortality in small populations of ungulates. Science 206:1101-1103, 1979.
Rossiter, P.B., Jessett, D.M. and Karstad, L.: Role of wildebeest fetal membranes and fluids in the transmission of malignant catarrhal fever virus. Vet. Rec. 113:150-152, 1983.
Watson, P.F.: Electroejaculation, semen characteristics and semen preservation of the brindled gnu. J. Reprod. Fertil. 47:123-126, 1976.
Wooding, F.B.: The role of the binucleate cell in ruminant placental structure. J. Reprod. Fertil. Suppl. 31:31-39, 1982.
Wurster, D.H. and Benirschke, K.: Chromosome studies in the superfamily Bovoidea. Chromosoma 25:152-171, 1968.
Zukowsky, L.: Bastarde zwischen Weißschwanz- und Streifengnu. Zool. Garten 33:165-173, 1967.