| 1 |
Brazilian Free-Tailed Bat
Tadarida brasiliensis (cynocephala)
Order: Chiroptera
Family: Molossidae
1) General Zoological Data
Free-tailed bats are called ‘free-tailed’ because of the lack of a membrane to its tail. They are widespread and classified under Molossidae (since the Eocene), a word derived from Greek molossos – a kind of wolf-dog because of the bats’ facial expression (Gotch, 1979). This group of bats is perhaps the most highly evolved of the microchiroptera, at least according to the studies on a very large material of specimens of Tadarida cynocephala by Wimsatt (1958). There are 16 genera and 86 species of Molossidae according to Nowak (1999) and they are widely distributed. Their evolutionary history is also detailed in that review. A much more detailed review of all chiroptera was published by Eger (2007) that often corrected inappropriately labeled specimens of past publications. This insectivorous species (often referred to as ‘house bat’) is widely distributed throughout the Southern North American States and occurs in huge numbers in the Texan ‘guano caves’ (Carlsbad Caverns in New Mexico). In the winter, most of the Tadarida brasiliensis migrate to Mexico and even further south. The animal weighs between 11 and 14 g and is frequently predated by owls, snakes, raccoons and other species, and is also a known carrier of rabies. Their longevity is 10-11 years (perhaps even 12 years) in the wild (Weigl, 2005). Two definite subspecies are known to exist in the USA (T.b.cynocephala & T.b.mexicana) which are distinguished by cranial morphology and they apparently interbreed; others have suggested that there are altogether nine subspecies comprised by this species. These bats are enormously important for the destruction of large numbers of moths. Thus, their diminution in recent years due to human interference is ecologically significant. The name tadarida is debated and poorly documented; ‘free-tail’ refers to the independence of the tail form the flight membrane (Gotch, 1979). The maintenance of insectivorous bats is difficult but it has been possible. Puschmann (1989; 2004) provided some details of such management in captivity, as did Stephens in some detail (1962).
 |
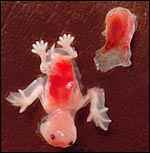
Free-tailed bat in flight (From Tuttle). |
 |
Free-tailed bat sitting (From Tuttle). |
 |
The mother of the first specimen (From R. Wilkins). |
2) General Gestational Data
The length of gestation is 77-90 days (Stephens & Easterbrook, 1969 indicated that it lasts 4 months) and singleton births are the rule. The fetuses gestate in the right uterine horn only and twins are extremely uncommon. The size of the naked neonates is about 25 mm (Hayssen, 1993). Stephens (1962) who has amassed the greatest database described it thus: “Total weight of mother and fetus was 15.2g, weight of the fetus 2.7 g, and weight of the placenta 0.8 g”. The placentas examined for the description here also weighed less than 1 g; in fact, we were unable to determine their precise weights, lacking an appropriate scale. Van der Merwe et al. (1986) who studied reproduction in the African Tadarida pumila, concluded that breeding is seasonal, that females are polyestrous, and that gestation lasts 60 and occurs in the right uterine horn.
The first specimen was kindly made available by Dick Wilkins, Poway, CA who is active in the ‘Bat Conservation International’ Organization. Ovulation is confined to the right ovary and occurs in late March; young arrive in June/July. Only one reproduction per year is usual and twins are exceptional. Neonates normally weigh up to 3-4 g and they nurse for ~5 weeks (Nowak, 1999; Hayssen et al., 1993). The placenta of this first specimen with a male fetus was very small and was fixation in alcohol. It had early degenerative changes (autolysis). Thus, the details are less than optimally preserved.
3) Implantation
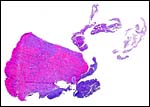
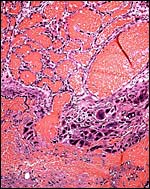
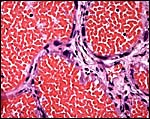
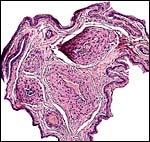
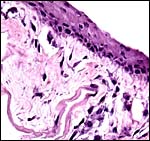
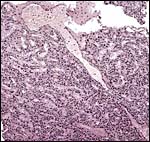
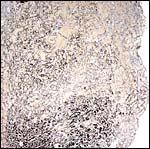
The earliest publication on this species’ placentation comes from Hamlett (1934), but thereafter Wimsatt (1958) provided a very large summary of his study of over 50 specimens in his general review of chiropteran placentation. He especially acknowledged the differences in the developmental stages of this species’ placentation (see below). Then, Stephens (1962) provided information on their placental structure, including histochemical observations and with significant notes on maintaining these bats in captivity. Next most importantly, Stephens & Cabral (1972) studied the fine structure of T. brasiliensis cynocephala and discussed the developmental differences in placental structure. Mossman (1973) thereafter described this placentation in some detail as well and found that the first placental attachment is anti-mesometrial. The placenta is a superficial diffuse, discoid endotheliochorial yolk sac placenta that evolves possibly into a hemochorial organ (Wimsatt, 1958; 1962) as the maternal vascular endothelium becomes significantly attenuated in later stages. Sansom (1932), however, identified the earliest implantations in the mid-portion and lateral to the mesometrium.
4) General Characterization of the Placenta
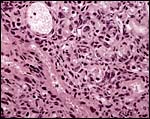
In his contribution of chiropteran placentas, Wimsatt (1958) stated: “It will be shown that the maternal capillary endothelium persists until late stages of pregnancy in all families studied with the probable exception of the Molossidae. The placenta is actually endotheliochorial, even in those families in which it has long been interpreted as hemochorial”. He then proceeded to show that a very thin ‘anuclear’ layer of thin endothelial remnants remains of the maternal capillary bed. According to him this is a ‘discoidal and labyrinthine placenta’. One of the most detailed descriptions of the placenta of free-tailed bats however comes from Stephens & Cabral (1972) who described it as a “diffuse labyrinthine endotheliodichorial placenta” and they did superb electron microscopy on this organ. In the following discussion, the two stages (early #1) and late (#2) are contrasted, as two different gestational ages became available nearly simultaneously. They are labeled #1 and #2 respectively.
Inasmuch as the first specimen under discussion was obtained in the middle of May, it would appear that its gestation is in its first trimester. The embryo measured 1.3 cm in length after fixation, and the specimen had been preserved in absolute alcohol.
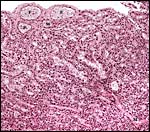
This is an endothelio-chorial placenta with areas of a labyrinthine structure and the implantation is ‘superficial’. Mossman describes it as being hemomonochorial in type, as studied electronmicroscopically. There are no lobulations or proper villous formations. The “maternal tubules are lined by ectoplasmic processes of trophoblast” (Stephens & Cabral, 1972, as quoted by Mossman).
5) Details of fetal/maternal barrier
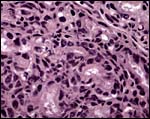
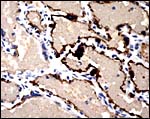
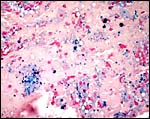
The initial yolk sac placentation is soon replaced and becomes an endotheliochorial structure. The displacement is accomplished by the developing allantois. Electron microscopic study of the yolk sac epithelium by Stephens & Easterbrook (1971) disclosed the presence of a cytoplasmic organelle (”membranous organelle”) in the endoderm that is assumed to function in the glycogen metabolism. In addition, there are many coated vesicles that these authors relate to an absorptive function of the endodermal epithelium. This function is subsumed by trophoblast later in gestation.
The details of the placentation in free-tailed bats are nearly all the result of the extensive investigations by Stephens et al. (1962 – 1971). Wooding & Flint (1994) illustrated (from Stephens, 1969) the electronmicroscopy of the continuous nature of the syncytiotrophoblastic attachment to the basement membrane and no direct connection to the maternal blood and Enders (1982) referred to the ‘cellular’ barrier as being hemomonochorial (results of Stephens’ study). Enders et al. (1998) again referred to the central labyrinthine region of the molossid bat placenta and illustrated the maternal blood channel as being lined by (mono)-cytotrophoblast having “intrasyncytial bays”. Cellular trophoblast gives rise to syncytium that penetrates the endometrium, destroys the endometrium and encircles maternal vessels. The endothelium and pericytes of these maternal vessels are left intact (Stephens & Cabral, 1972). Eventually, the maternal component becomes extremely thin and is then apparently anuclear. A prominent feature is the seeming infiltration of the fetal capillaries into the syncytium.
6) Umbilical cord
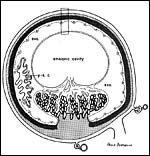
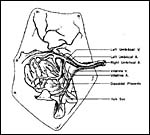
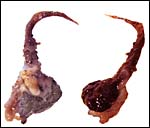
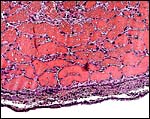
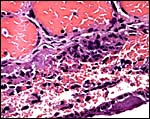
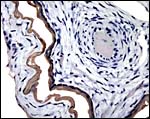
An extensive consideration of the cord structure is provided by Stephens (1962) and its sketch has been reproduced above. The cord contains 5 vessels which, according to this careful observer’s description are as follows: “...a vitelline artery which comes from the dorsal aorta…, a vitelline vein which enters the vena cava…, a right and left umbilical artery, and the left umbilical vein”. He also observed that, at term, the left umbilical vein and right umbilical artery are much larger, as can be seen in the following illustration. These are responsible for the ‘entire blood supply to the discoidal placenta’. The left umbilical artery supplies the chorion outside of the disk. The length of the umbilical cord has not been referred to and it was too short in these specimens to be measured after fixation.
7) Uteroplacental circulation
Large maternal channels are opened early in gestation around which the fetal capillary network of the labyrinth is congregated (Stephens, 1962). At this site, the endometrium degenerates, forms hemosiderin and makes giant cells. Deeper invasion by trophoblast does not take place. No observations have been described other than those published by Wimsatt (1958) and Stephens (1962). Wimsatt showed that the maternal arteries penetrate (initially in parallel structures) from the uterus to the fetal side of the disk and discharge the blood into a tortuous system of intercommunicating tubules that lead back to the decidual portion of the uterus where they coalesce to form irregular sinus-like blood spaces and Stephens described a method with which he injected fetal vessels.
8) Extraplacental membranes
There is no decidua capsularis but some decidua basalis develops and is destroyed in the superficially invasive trophoblast. A bilaminar omphalopleure is established in early development and the yolk sac is large. A vascularized mesoderm dissects between the two epithelial layers and both remain intact. When the yolk sac has increased to being 2.5 mm in size, the vascularized allantois begins its development. Details have been described by Stephens (1962). The amnion develops by folding, but Mossman has it as forming by cavitation.
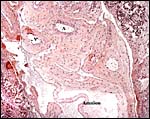 |
Portion of the umbilical cord in the empty sac (#1) with amnion and major fetal vessels. |
9) Trophoblast external to barrier
Stephens (1962) commented specifically that there is no deep invasion of trophoblast.
10) Endometrium
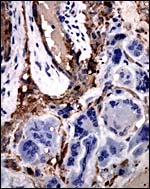
The uterus is bicornuate with the right horn being larger and the one to gestate the fetus.
Stephens (1962) has given a detailed description of the features of uterus, endometrium and associated features. Although a true decidua is not mentioned, Stephens (1962) refers to the initial ‘trophoblastic pad’ as consuming the initial decidua that later mostly degenerates (‘plasmolyzes’). Interestingly, of 300 gestations studied by Sherman (1937), only one was found in the left uterine horn. Of note is the squamous nature of the endometrial lining identified by this author. While he also stated that the horns are nearly equal in size, during early gestation the right horn enlarges specifically and is nearly exclusively used for placentation. Only Sherman (1937) has once, in 300 cases, found left implantation. A decidua capsularis is not formed.
11) Various features
None remarkable features develop according to Stephens’ extensive study.
12) Endocrinology
Only the right ovary is employed and usually possesses a single corpus luteum - rarely two corpora (Mossman & Duke, 1973). Stephens (1962) has speculated on the possible reasons for this feature of asymmetrical usage; he thought that either local diffusion of progesterone from the corpus luteum or, more likely, differential blood flow can explain this. The left ovary is considerably smaller. Wimsatt (1979) has also discussed at length the asymmetry of ovarian and uterine employment for gestation in a variety of bat species; he concluded that ‘dextral dominance is most frequent’. Ovulation apparently occurs preferentially in the right ovary and the right uterine horn is larger. The ovary has a complete bursa with a ‘pore-like’ opening; it has a large rete ovarii and thin thecal gland with very sparse interstitial gland tissue, according to Mossman & Duke (1973). They also indicated that ovulation occurs only on the right side which was further elaborated upon by Jerrett (1979) who also provided some histochemical endocrine information on ovary and Leydig cells. The periodicity of Leydig cell activity (prominence) was described by Aoki (1997); it can be activated by hCG.
13) Genetics
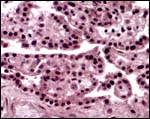
This species has 48 chromosomes, as displayed below. The karyotypes shown below come from Hsu & Benirschke, 1970 and they were then gifts of Dr. R.J. Baker in Texas. They came from specimens collected in Oklahoma. The Y-chromosome is the smallest acrocentric element.
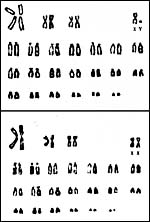 |
Karyotypes of male and female free-tailed bats. |
Caire & Haydari (1990) described the absence of a distinct fetal hemoglobin. The genetic population structure was studied by McCracken et al. (1994). Hybrids between the two subspecies only are known from the wild. The potential dispersal of bat migratory groups was studied with mtDNA and banding by Russell et al. (2005). No specific patterns were detected but the methodology was highlighted as being potentially useful for further studies.
14) Immunology
Transplacental rabies transmission was suggested by Ramachandran & Jayakumar (1996).
15) Pathological features
Clark et al. (1996) investigated deaths from rabies and pesticides. Similarly, Geluso et al. (1981) described pesticide residues in these bats. Other metal residues were detailed by Thies & Gregory (1994). The transmission of rabies virus is the principal concern regarding bats, especially those found dead by the public. Conventionally these are submitted to the County Veterinary Office for testing. It has also been demonstrated that rabies virus can pass the placenta and infect the fetus (Steece & Calisher, 1989). More recently, the possible transmission of the West Nile virus was studied by Davis et al. (2005). While rarely antibodies were detected, no viral transmission appeared to be likely by these bats. Transplacental transfer of lipophilic organochlorine pesticides was shown by Thies & McBee (1994). Flea larvae were identified by Elbel & Bossard (2007).
16) Physiologic data
Kunz & Robson (1995) reported on birth size and growth rates. The reasons for the right-sided ovulation are unknown. The process of erythropoiesis and vasculogenesis in the yolk sac from ‘stem cells’ was described in meticulous detail including EM by Stephens & Cabral-Anderson (1976). Kleinschmidt et al. (1987) described the composition of the two components of hemoglobin. Kunz et al. (1995) provided details of milk composition during daily cycles of several bat species. The varied food intake (high fat content of insects) leads to high levels of cholesterol but low levels of triglycerides but did not lead to atherosclerosis (Kunz et al., 1996). Vater & Siefer (1995) studied the details of the bat cochlea in great detail and with respect to the sonar reception system. Hermanson & Wilkins (2008) studied the bone formation during bat neonatal development and found more rapid development of hind limbs. Blood lipids and cholesterol have been studied by Widmaier et al. (1996).
17) Other resources
Numerous web sites can be accessed and these contain an enormous amount of additional information for this important species. No cell lines are available.
18) Other remarks – What additional Information is needed?
More details of the distribution of trophoblast with modern staining techniques would be welcomed. Cell lines for the “Frozen Zoo” at CRES in San Diego are badly needed, as are those of other bat species.
Acknowledgement
The first two animal photographs in this chapter come from the website of Dr. Merlin D. Tuttle on free-tailed bats. The second specimen of bat placenta was kindly made available by Drs. Deborah Kemmerer Cottrell and Manu Sebastian from Texas. The animal photographs and the entire first specimen come from Dick Wilkins, Poway, CA 92064. I am most grateful for their help.
References
Aoki, A.: Seasonal and experimental reactivation of Leydig cells of the bat Tadarida brasiliensis. Biocell. 21:19-28, 1997.
Caire, W. and Haydari, K.: Absence of electrophoretically distinct fetal hemoglobins in the bats Tadarida brasiliensis and Lasiurus borealis. J. Mammal. 71:695-697, 1990.
Capanna, E. and Civitelli, M.V.: Chromosomal mechanisms in the evolution of chiropteran karyotypes. Chromosomal tables of Chiroptera. Caryologia 23:79, 1970.
Clark, D.R. Jr., Lollar, A. and Cowman, D.F.: Dead and dying Brazilian free-tailed bats (Tadarida brasiliensis) from Texas: Rabies and pesticide exposure. Southwest Nat. 41:275-278, 1996.
Davis, A., Bunning, M., Gordy, P., Panella, N., Blitvich, B. and Bowen, R.: Experimental and natural infection of North American bats with West Nile virus. Amer. J. Trop. Med. Hyg. 73:467-469, 2005.
Eger, J.L.: Family Molossidae. Pp. 399-438, in A.L. Gardner, ed.: Mammals of South America, Volume 1. University of Chicago Press, 2007.
Elbel, R.E. and Bossard, R.L.: Observations and larval descriptions of fleas (Siphonaptera: Ceratophyllidae, Ctenophthalmidae, Ishnopsyllidae) of the southern flying squirrel, little brown bat, and Brazilian free-tailed bat (Mammalia: Rodentia, Chiroptera). J. Med. Entomol. 44:915-922, 2007.
Enders, A.C.: Whither studies of comparative placental morphology? J. Reprod. Fert. Suppl. 31:9-15, 1982.
Enders, A.C., Blankenship, T.N., Lantz, K.C. and Enders, S.S.: Morphological variation in the interhemal areas of chorioallantoic placentae. A review. Trophobl. Res. 12:1-19, 1998.
Geluso, K.J., Altenbach, J.S. and Wilson, D.E.: Organochlorine residues in young Mexican freetailed bats from several roosts. Amer. Midl. Nat. 105:249-257, 1981.
Gotch, A.F.: Mammals – Their Latin Names Explained. Blandford Press, Poole, Dorset, 1979.
Hamlett, G.W.D.: Implantation und Embryonalhűllen bei zwei sűdamerikanischen Fledermäusen. 79:146-149, 1934.
Hayssen, V., van Tienhoven, A. and van Tienhoven, A.: Asdell’s Patterns of Mammalian Reproduction: a Compendium of Species-specific Data. Comstock/Cornell University Press, Ithaca, 1993.
Hermanson, J.W. and Wilkins, K.T.: Growth and development of two species of bats in a shared maternity roost. Cells Tissues Organs 187:24-34, 2008.
Hsu, T.C. and Benirschke, K.: An Atlas of Mammalian Chromosomes. 4, Folio 159, 1970.
Jerrett, D.P.: Female reproductive patterns in nonhibernating bats. J. Reprod. Fertil. 56:369-378, 1979.
Kleinschmidt, T., Rücknagel, K.P., Weber, R.E., Koop, B.F. and Braunitzer, G.: Primary structure and functional properties of the hemoglobin from the free-tailed bat Tadarida brasiliensis (Chiroptera). Small effect of carbon dioxide on oxygen affinity. Biol. Chem. Hoppe Seyler 368:681-690, 1987.
Kunz, T.H., Oftedal, O.T., Robson, S.K., Kretzmann, M.B. and Kirk, C.: Changes in milk composition during lactation in three species of insectivorous bats. J. Comp. Physiol. [B]. 164:543-551, 1995.
Kunz, T.H. and Robson, S.K.: Postnatal growth and development in the Mexican free-tailed bat (Tadarida brasiliensis mexicana): Birth size, growth rates, and age estimation. J. Mammal. 76:769-783, 1995.
McCracken, G.F., McCracken, M.K. and Vawter, A.T.: Genetic structure in migratory populations of the bat Tadarida brasiliensis mexicana. J. Mammal. 75:500-514, 1994.
Merwe, M. van der, Rautenbach, I.L. and Colf, W.J. van der: Reproduction in females of the little free-tailed bat, Tadarida (Chaerephon) pumila, in the eastern Transvaal, South Africa. J. Reprod. Fertil. 77:355-364, 1986.
Mossman, H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Mossman, H.W. and Duke, K.L.: Comparative Morphology of the Mammalian Ovary. University of Wisconsin Press, Madison, Wisconsin, 1973.
Nowak, R.M.: Walker’s Mammals of the World. 6th ed. The Johns Hopkins Press, Baltimore, 1999.
Puschmann, W.: Zootierhaltung. Vol. 2, Säugetiere. VEB Deutscher Landwirtschaftsverlag Berlin, 1989.
Puschmann, W.: Zootierhaltung. Tiere in menschlicher Obhut. Säugetiere. Verlag Harri Deutsch, Frankfurt, 2004.
Ramachandran, S. and Jayakumar, R.: Transplacental transmission in rabies. Ind. Vet. J. 73:108-110, 1996.
Russell, A.L., Medellin, R.A. and McCracken, G.F.: Genetic variation and migration in the Mexican free-tailed bat (Tadarida brasiliensis mexicana). Mol. Ecol. 14:2207-2222, 2005.
Samson, G.S.: Notes on some early blastocysts of the South American bat Molossus. Proc. Zool. Soc. London, Part 1:113-118, 1932.
Sherman, H.B.: Breeding habits of the free-tailed bat. J. Mammal. 18:183-184, 1937.
Steece, R.S. and Calisher, C.H.: Evidence for prenatal transfer of rabies virus in the Mexican free-tailed bat (Tadarida brasiliensis Mexicana). J. Wildl. Dis. 25:329-334, 1989.
Stephens, R.J.: Histology and histochemistry of the placenta and fetal membranes in the bat, Tadarida brasiliensis cynocephala (with notes on maintaining pregnant bats in captivity). Amer. J. Anat. 111:259-275, 1962.
Stephens, R.J.: The development and fine structure of the allantoic placental barrier in the bat Tadarida brasiliensis cynocephala. J. Ultrastruct. Res. 28:371-398, 1969
Stephens, R.J. and Cabral, L.: Direct contribution of the cytotrophoblast to the syncytiotrophoblast in the diffuse labyrinthine endothelio-chorial placenta of the bat. Anat. Rec. 169:243-252, 1971.
Stephens, R.J. and Cabral, L.: Cytological differentiation of the mesothelial cells of the yolk sac of the bat, Tadarida brasiliensis cynocephala. Anat. Rec. 171:293-312, 1971.
Stephens, R.J. and Cabral, L.: The diffuse labyrinthine endotheliodichorial placenta of the free-tail bat: A light and electron microscopic study. Anat Rec. 172:221-252, 1972.
Stephens, R.J. and Easterbrook, N.: A new cytoplasmic organelle, related to both lipid and glycogen storage materials in the yolk sac of the bat, Tadarida brasiliensis cynocephala. Amer. J. Anat. 124:47-56, 1969.
Stephens, R.J. and Easterbrook, N.: Development of the cytoplasmic membranous organelle in the endodermal cells of the yolk sac of the bat, Tadarida brasiliensis. J. Ultrastruct. Res. 24:239-248, 1968.
Stephens, R.J. and Easterbrook, N.: A new cytoplasmic organelle related to both lipid and glycogen storage materials in the yolk sac of the bat, Tadarida brasiliensis. Amer. J. Anat. 124:47-56, 1969.
Stephens, R.J. and Easterbrook, N.: Ultrastructural differentiation of the endodermal cells of the yolk sac of the bat, Tadarida brasiliensis. Anat. Rec. 169:207-242, 1971.
Thies, M. and McGregor, D.: Residues of lead, cadmium, and arsenic in livers of Mexican free-tailed bats. Bull. Environ. Contam. Toxicol. 52:641-648, 1994.
Thies, M.L. and McBee, K.: Cross-placental transfer of organochlorine pesticides in Mexican free-tailed bats from Oklahoma and New Mexico. Arch. Environm. Contam. Toxicol. 27:239-242, 1994.
Vater, M. and Siefer, W.: The cochlea of Tadarida brasiliensis: Specialized functional organization in a generalized bat. Hear. Res. 91:178-195, 1995.
Weigl, R.: Longevity of Mammals in Captivity from the Living Collections of the World. E. Schweizertbart’sche Verlagsbuchhandlung (Nägele und Obermiller), Stuttgart, 2005.
Widmaier, E.P., Gornstein, E.R., Hennessey, J.L., Bloss, J.M., Greenberg, J.A. and Kunz, T.H.: High plasma cholesterol, but low triglycerides and plaque-free arteries in Mexican free-tailed bats. Amer. J. Physiol. 271 (5 Pt 2):R1101-1106, 1996.
Wimsatt, W.A.: The allantoic placental barrier in chiroptera: A new concept of its organization and histochemistry. Acta Anat. 32:141-186, 1958.
Wimsatt, W.A.: Some aspects of the comparative anatomy of the mammalian placenta. Amer. J. Obstet. Gynecol. 84:1568-1594, 1962.
Wimsatt, W.A.: Reproductive asymmetry and unilateral pregnancy in Chiroptera. J. Reprod. Fertil. 56:345-357, 1979.
Wooding, F.B.P. and Flint, A.P.F.: Placentation. Chapter 4 (pp.233-460) in, G.E. Lamming, ed. Marshall’s Physiology of Reproduction, 4th ed. Vol. 3, Part 1. Chapman & Hall, London, 1994.