| (Clicking
on the thumbnail images will launch a new window and a larger version
of the thumbnail.) |
| Last updated: Jan 31, 2006. |
Rhynchocyon petersi
With Alisa L. Newton, VMD
Order: Macroscelidea
Family: Marcoscelididae
1) General Zoological Data
Since the revision of elephant-shrews by Corbet and Hanks (1968), 15 species now exist in this family of mammals. They are currently considered to be a single line of animals of the ancient but newly created super-order of Afrotheria. Afrotheria comprise elephant, hyrax, sea cow, aardvark, elephant shrew, golden-mole and tenrec. The former notion of associating them with insectivores is no longer considered to be valid but it must also be cautioned that the reassignment to Afrotheria is only tentative and not absolutely confirmed. Indeed, Szalay (1977) placed them as having a common stock with lagomorphs and some of my findings suggest that this may be not far off the mark.
There are three species in this smaller subfamily of giant elephant-shrews, the genus Rhynchocyon . A comprehensive symposium on elephant shrews was edited by Perrin (1995); it comprises many important aspects of their biology. In addition, numerous references are available from a web site on ‘Elephant-shrews or Sengis' that is edited by G. Rathbun. These animals are not commonly seen in zoos and, by some authors, they were regarded as pests even though many are now comprised among the severely endangered species of small mammals. This species comes from Kenya and Tanzania where the animals live primarily in wooded areas. This is the largest elephant shrew and remarkable because of the long, movable snout and long legs (the reason for the name Macroscelidea). In the Bantu language they are referred to as ‘sengi(s)', a name even now often employed for these animals. More properly though they are here referred to as black and rufous giant elephant shrews, also as ‘checkered elephant-shrews'. The animals weigh between 300 and 550 g, live 4-5 years and have one or two young. A most successful breeding colony is maintained at the Philadelphia Zoo. Baker et al. (2005) provided a review of their colony that is worth reading.
 |
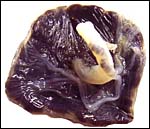
Giant Elephant Shrew ( California Academy of Sciences) |
A pair of giant elephant shrews (California Academy of Sciences). |
|
2) General Gestational Data
Elephant shrews live as monogamous pairs in a densely wooded habitat. Litter size is one or two offspring that weigh around 10 g, and implantation occurs 7-9 days after coitus. The gestational length is probably around 50 days, but definitive studies have not been found. The placenta of this singleton pregnancy weighed 4.1 g and measured 3.5 x 2.5 x 0.5 cm. There have been 14 offspring in the Philadelphia Zoo colony that began with two pairs of captive bred animals in 2000. Neonates are naked and are deposited into a nest which they leave in 3-4 weeks.
3) Implantation
No studies of early implantation have been reported on Rhynchocyon petersi . In Elephantulus it has been described as occurring 7-9 days post copulation and Oduor-Okelo et al. (2004) have described the placenta of Petrodomus tetradactylus in admirable detail.
4) General Characterization of the Placenta
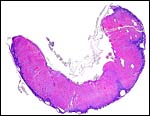
The elephant shrews have a long duplex uterus in which the blastocyst implants mesometrially and superficially. It is a discoid, hemochorial organ with a labyrinthine architecture. There is a large, permanent allantoic sac. The only relevant publications on the placentas of Rhynchocyon chrysopygus have been by Oduor-Okelo (1984, 1985) who had the opportunity of examining 17 pregnant uteri of that species. The findings of that study are essentially similar to the placenta here described.
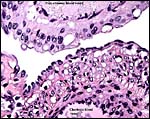
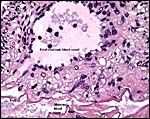
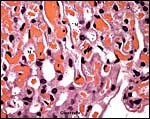
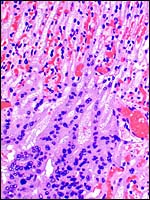
This placenta is remarkable in some respects that are not yet fully understood. Please especially note the architecture of the large, free surface vessels on the fetal aspect of the placenta. They are not only unusually prominent but have a remarkable histologic appearance (see below). In addition to the large superficial vessels that come from the cord and that have a prominent epithelial cover, there are smaller chorionic vessels that lack this epithelial investment. Similarly, the intrachorial vessels do not have the epithelial surface investment. When these epithelial cells that Oduor-Okelo (1984) described as “squamous” are traced along the allantoic membrane, they are found to cover its periphery as well. The large amount of cytoplasm of these cells is finely vacuolated and also has some larger vacuoles; when stained with PAS, the vacuoles are empty. Silver stains show that the nuclei of these epithelial cells stain positively, contrary to the intrachorial fetal vessels that do not possess this epithelial investment but have some of the larger vacuoles. Those vessels do not contain lipid, however. The epithelial cells are tentatively interpreted as being endodermally-derived allantoic epithelium. The cells are generally columnar, the nuclei have irregular polarity and many nuclei are degenerating. Some of these unusual epithelial cells have multiple nuclei and two types of vacuoles. The larger vacuoles lack lipid content but the numerous small vacuoles are filled with oil red O-staining lipids. The vacuoles are PAS and silver-stain negative, while the nuclei stain with Gomori's silver stain. They are altogether quite an unusual feature of the elephant-shrew placenta and the function of these epithelial cells is unclear at this time. Nevertheless, when one compares the macroscopic features of the placental surface with that of the rabbit, they are very similar. Not only that, there are also some microscopic similarities although the epithelial cells of rabbits are much less striking.
5) Details of fetal/maternal barrier
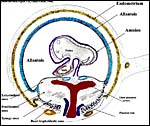
Mossman referred to the placenta of elephant-shrews as being labyrinthine but without lobulation, and having a hemochorial barrier arrangement. The placenta studied here, however, shows a mild degree of lobulation. There are a thick trophospongium and possibly some superficially invasive trophoblastic giant cells. The trophoblast is single-nucleated; syncytial cells on the labyrinthine structures were not evident. The thick chorioallantois has an epitheliochorial relationship to the endometrium. The allantoic cavity remains to term and is connected to the bladder by the urachus. The principal features of the placental/membrane relationships are sketched diagrammatically below and are also well illustrated in the study on Petrodomus tetradactylus (Oduor-Okelo et al., 2004).
6) Umbilical cord
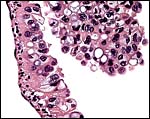
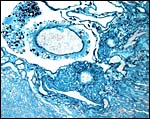
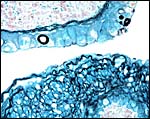
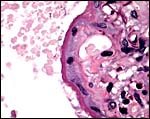
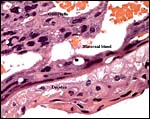
The cord was eccentrically inserted and measured 2 x 0.3 cm, had no spirals and contained a very markedly coiled vessel. Its surface was smooth but there was mild squamous metaplasia on its surface. Data on cord length in other elephant-shrews are only available for Elephantulus fuscipes (Spatz, 1968). He lists 2.5 - 2.9 cm as the length at term. The umbilical cord contains two arteries and one vein, plus the allantoic duct that is located between the two arteries.
 |
Cross sections through the umbilical cords. Allantoic duct at arrow. |
 |
Surface of the umbilical cord with slight squamous metaplasia. |
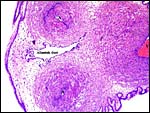 |
The allantoic duct is in between the two umbilical arteries. |
7) Uteroplacental circulation
No significant studies have been undertaken to my knowledge, but there are large maternal sinusoids coursing through the placental labyrinth that are well depicted in the photomicrographs. It is essential that implanted placentas of Rhynchocyon petersi be studied, as was done with Petrodomus tetradactylus (Oduor-Okelo et al., 2004).
8) Extraplacental membranes
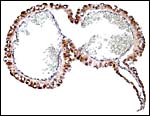
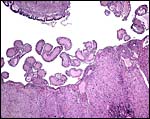
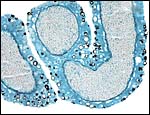
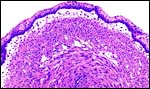
A permanent allantoic sac is present and the degenerating yolk sac remains is connected to term to the intestines.
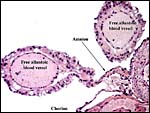
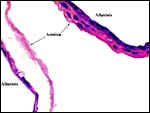 |
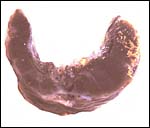
One portion of membranes with amnion in the center and allantoic membrane outside. |
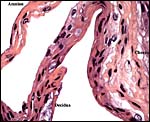 |
This is from the edge of the placenta (which is at the right) and the membranes extend to the left. |
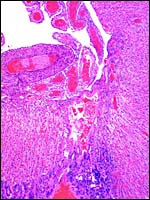
9) Trophoblast external to barrier
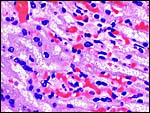
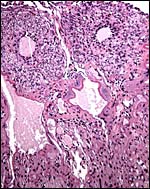
The central portion of the placenta has a large deposit of trophoblastic giant cells surrounding the maternal sinuses, but there is only very minimal invasion of the basal decidua. It is unknown whether there is trophoblastic invasion of the myometrium. It is thus essential that implanted placenta are studied in great detail. This was done in the study of Petrodomus tetradactylus (Oduor-Okelo, 2004) and they described well-developed ‘mesoplacenta' that I believe is somewhat similar to that of rabbits.
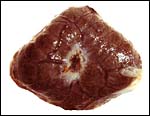
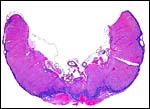
Maternal aspect of placenta with large maternal sinuses and trophoblastic giant cells. |
|
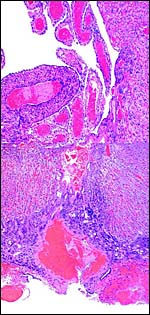
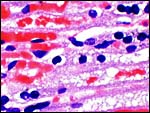
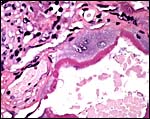
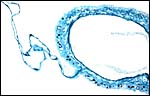
10) Endometrium
The uterus is duplex in Elephantulus (Mossman, 1987) and a decidua develops during gestation. At least the elephant shrew investigated by v.d. Horst & Gilman (1941) ( Elephantulus myurus jamesoni ) has periods of menstruation.
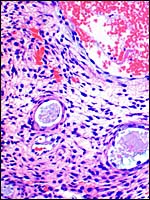 |
Placental floor with minimal decidua (two endometrial glands are seen in cross section). |
11) Various features
No unusual features are known to me.
12) Endocrinology
Mossman & Duke (1973) remarked that the related Elephantulus has an unusually high location of the ovaries and that it ovulates ~50 follicles, for reasons unknown. These stem from unifollicular ova, unlike some other species and they suggested that, as in Viscacha, their gonadotropin secretion needed to be investigated.
13) Genetics
After the original suggestion of Springer et al. (1997, 1997) to separate some endemic African mammals from former alliances, Murphy et al. (2001) reviewed the phylogenetics of the Afrotheria in some greater detail. Thus, a new Order was created and many animals were assigned to different ancestries. These concepts, especially the notion of Afrotheria, are now widely accepted and much new information on gene comparisons has been published. Robinson et al. (2004), using chromosome painting comparisons of different species, thus placed aardvark and elephant-shrews as sister species. Hedges (2001) has written an interesting commentary on the plate tectonic aspects of mammalian evolution, specifically about Afrotheria.
No elephant shrew hybrids have been reported and only few chromosome studies have been done. Wenhold & Robinson (1987) did the first study of three species of elephant-shrews with banding and silver staining methods. They found that Petrodomus tetradactylus had 2n= 28; Elephantulus rupestris had 2n=26, and Macroscelides proboscides had 2n=26 elements. In their figure 4 these authors compared the structural dissimilarities of several elements in great detail and decried the fact that chromosomal rearrangements are generally undervalued in taxonomic comparisons of related species. They considered chromosomal fusion as an important mechanism of evolution. In another contribution on Elephantulus species and the four-toed Petrodromus tetradactylus , the authors (Tolliver et al., 1989) also found low chromosome numbers; the latter also having 2n=28 (16 metacentrics, 10 acrocentrics).
14) Immunology
To the best of my knowledge this has not been studied.
15) Pathological features
Hoopes and Montali (1980) reported frequently recurring inflammation and tail necrosis in a captive colony of Elephantulus rufescens . Some animals also had necrosis of ear tips. The lesions were beautifully illustrated and were attributed to crowding and tail-biting, with infection being secondary events.
16) Physiologic data
An important feature of elephant shrews is their dependence on smells, especially for sex identification (Koontz & Roeper, 1983 – see also ‘Small Mammals'). The scent is secreted by extensive sternal glands. They have a largely crepuscular activity pattern and are primarily insectivorous. Elephant shrews have a cecum.
17) Other resources
No further resources are known to me.
18) Other remarks – What additional Information is needed?
Clearly, additional specimens and especially from younger gestations are needed as well as implanted placentas. The nature of the epithelial cells on the allantoic surface vessels requires physiological study. Chromosome studies are desirable.
Acknowledgement
I am most grateful to Alisa L. Newton, V.M.D. of the Philadelphia Zoo for this material. The animal photographs in this chapter come from the Internet's collection by the Academy of Sciences .
References
Baker, A.J., Lengel, K., McCafferty, K. and Hellmuth, H.: Black-and-rufous sengi (Rhynchocyon petersi ) at the Philadelphia Zoo. Afrotherian Conservation. Newsletter of the IUCN/SSC Afrotheria Specialist Group. # 3:6-7, 2005. (Available from the internet).
Corbet, G.B. and Hanks, J.: A revision of the elephant-shrews, Family Macroscelididae. Bull. Brit. Mus. Natural Hist. Zoology 16:47-111, 1968.
Hedges, S.B.: Afrotheria: Plate tectonics meets genomics. ‘Commentary'. PNAS 98:1-2, 2001.
Hoopes, P.J. and Montali, R.J.: Tail lesions in captive elephant shrews. Pp. 425-430 in, The Comparative Pathology of Zoo Animals. R.J. Montali and G. Migaki eds., Smithsonian Institution Press, Washington D.C. , 1980.
Horst, C.J. van den and Gilman, J.: The menstrual cycle in elephantulus. S. Afr. J. Med. Sci. 6:27-47, 1941.
Koontz, F.W. and Roeper , N.J. : Elephantulus rufescens. Mammalian Species # 204: 1-5, 1983.
Mossman, H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Mossman, H.W. and Duke, K.L.: Comparative Morphology of the Mammalian Ovary. University of Wisconsin Press, Madison , Wisconsin , 1973.
Murphy, W.J., Eisirik, E., O'Brien, S.J., Madsen, O., Scally, M., Douady, C.J., Teeling, E., Ryder, O.A., Stanhope, M.J., de Jong, W.W. and Springer, M.S.: Resolution of the early placental mammal radiation using Bayesian phylogenetics. Science 294:2348-2351, 2001.
Oduor-Okelo, D.: Histology of the chorioallantoic placenta of the golden-rumped elephant shrew (Rhynchocyon chrysopygus Gunther, 1881). Anat. Anz. 157:395-407, 1984.
Oduor-Okelo, D.: Ultrastructural observations on the chorioallantoic placenta of the golden-rumped elephant shrew, Rhynchocyon chrysopygus . Afr. J. Ecol. 23:155-166, 1985.
Oduor-Okelo, D. Gombe, S. and Amoroso, E.C.: The placenta and fetal membranes of the short-nosed elephant shrew, Elephantulus rufescens (Peters, 1878). Säugetierk. Mitt. 28:293-301, 1980.
Oduor-Okelo, D., Katema, R.M. and Carter, A.M.: Placenta and fetal membranes of the four-toed elephant-shrew Petrodomus tetradactylus. Placenta 25:803-809, 2004.
Perrin, M.R., ed. The Biology of Elephant-shrews. A symposium held during the 6 th International Theriological Congress, Sydney, July 5, 1993 . Mammal Review - Volume 25: No. 1&2.
Robinson, T.J., Fu, B., Ferguson-Smith, M.A. and Yang, F.: Cross-species chromosome painting in the golden mole and elephant-shrew: support for the mammalian clades Afrotheria and Afroinsectiphillia but not Afroinsectivora. Proc. Roy. Soc. London B 271:1477-1484, 2004.
Small Mammals. Fact sheet produced by Smithsonian National Zoological Park . Available from the internet.
Spatz, W.B.: Nabelschnur-Längen bei Insektivoren und Primaten. Z. Säugetierk. 33:226-239, 1968.
Springer, M.S., Cleven, G.C., Madsen, O., de Jong, W.W., Waddell, V.G., Amrime, H.M. and Stanhope, M.J.: Endemic African mammals shake the phylogenetic tree. Nature 388:61-64, 1997.
Springer, M.S., Amrime, H.M., Burk, A. and Stanhope, M.J.: Additional support for Afrotheria and Paenungulata, the performance of mitochondrial versus nuclear genes, and the impact of data partitions with heterogeneous base composition. Syst. Biol. 48:65-75, 1999.
Szalay, F.S.: Phylogenetic relationships and a classification of eutherian Mammalia. In, M.K. Hecht, P.C. Goody and M.B. Hecht, eds. Major Patterns in Vertebrate Evolution pp. 115-174. Plenum Press, N.Y. 1977.
Tolliver, D.K., Robbins, L.W., Rautenbach, I.L., Schlitter, D.A. and Coetze, C.G.: Biochemical systematics of elephant shrews from southern Africa . Biochem. System. Ecol. 17:345-355, 1989.
Wenhold, B.A. and Robinson, T.J.: Comparative karyology of three species of Elephant-shrew (Insectivora: Macroscelididae). Z. Säugetierk. 52:1-9, 1987.