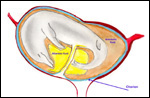
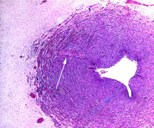
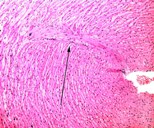
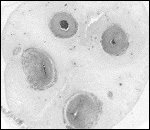
| |
Acknowledgement
Specimens were largely obtained through the courtesy of Dr. S.H. Ridgway,
San Diego.
References
Benirschke, K., Johnson, M.L. and Benirschke, R.J.: Is ovulation in dolphins,
Stenella longirostris and Stenella attenuata, always copulation-induced?
Fishery Bull. 78:507-528, 1980.
Benirschke,
K.: Past and future investigations to enhanced understanding of cetaceans.
In, Bottlenose Dolphin Reproduction Workshop. San Diego, California June
3-6, 1999. D. Duffield and T. Robeck, eds. AZA Marine Mammal Taxon Advisory
Group, Silver Spring, MD, 2000.
Bonn,
W. van: Infectious diseases and late abortions. pp. 279-287, In, Bottlenose
Dolphin Reproduction Workshop. San Diego, California June 3-6, 1999. D.
Duffield and T. Robeck, eds. AZA Marine Mammal Taxon Advisory Group, Silver
Spring, MD, 2000.
Brook,
F.: Ultrasound diagnosis of anencephaly in the fetus of a bottlenose dolphin
(Tursiops aduncas). J. Zoo Wildlife Med. 25:569-574, 1994.
Cowan,
D.F.: Pathological evidence of reproductive disease and/or dysfunction
in wild cetaceans, pp. 175-182, In, Bottlenose Dolphin Reproduction Workshop.
San Diego, California June 3-6, 1999. D. Duffield and T. Robeck, eds.
AZA Marine Mammal Taxon Advisory Group, Silver Spring, MD, 2000.
Duffield, D.A. , Ridgway, S.H. and Sparkes, R.S.: Cytogenetic studies
of two species of porpoise. Nature 213:189, 1967.
Duffield,
D. and Robeck, T. eds.: Bottlenose Dolphin Reproduction Workshop. San
Diego, California June 3-6, 1999. Marine Mammal Taxon Advisory Group,
Silver Spring, MD, 2000
Gray,
K.N. and Conklin, R.H.: Multiple births and cardiac anomalies in the bottle-nosed
dolphin. J. Wildl. Dis. 10:155-157, 1974.
Harrison,
R.J. and Ridgway, S.H.: Gonadal activity in some bottlenose dolphin (Tursiops
truncatus). J. Zool. London 165:355-366, 1971.
Hsu, T.C. and Benirschke, K.: An Atlas of Mammalian Chromosomes. Vol. 7, Folio 331, 1973.
Miller,
W.G., Adams, L.G., Ficht, T.A., Cheville, N.F., Payeur, J.P., Harley,
D.R., House, C. and Ridgway, S.H.: Brucella-induced abortion and infection
in bottlenose
dolphins (Tursiops truncatus). J. Zoo and Wildlife Med. 30:100-110,
1999.
Miller,
D.L. and Bossart, G.D.: Reproductive and fatal neonatal diseases in cetaceans
from Florida Oceanaria 1979-1999. pp. 183-186, In, Bottlenose Dolphin
Reproduction Workshop. San Diego, California June 3-6, 1999. D. Duffield
and T. Robeck, eds. AZA Marine Mammal Taxon Advisory Group, Silver Spring,
MD, 2000.
Mossman,
H.W.: Vertebrate Fetal Membranes. Comparative Ontogeny and Morphology;
Evolution; Phylogenetic Significance; Basic Functions; Research Opportunities.
The MacMillan Press, Ltd. Houndmills, 1987.
Nikaido,
M., Rooney, A.P. and Okada, N.: Phylogenetic relationships among certartiodactyls
based on insertions of short and long interspersed elements: Hippopotamuses
are the closest extant relatives of whales. Proc. Natl. Acad. Sci. USA
96:10261-10266, 1999.
Nowak,
R.M. and Paradiso, J.L.: Walker's Mammals of the World. 4th ed. Vol. II.
The Johns Hopkins University Press, Baltimore and London, 1983.
Pabst,
D.A., Rommel, S.A., McLellan, W.A., Williams, T.M. and Rowles, T.K.: Thermoregulation
of the intra-abdominal testes of the bottlenose dolphin (Tursiops truncatus)
during exercise. J. Exp. Biol. 198:221-226, 1995.
Perrin,
E.V.D., Benirschke, K. and Perrin W.F.: Monstrous dolphins - Malformations
in marine mammals. Teratology 39:p.51, abstr. # 472, 1989.
Prasad,
N., Mumford, D.M., Barsales, P.B., Whitman, T. and Wilbur, J.R.: Cytogenetic
studies of dolphin (Tursiops truncatus) by an extended tissue culture
technique. Experientia 26:1167, 1968.
Reddy,
M., Echols, S., Finklea, B., Busbee, D., Reif, J. and Ridgway, S.H.: PCBs
and chlorinated pesticides in clinically healthy Tursiops truncatus:
Relationships between levels in blubber and blood. Marine Pollution Bull.
36:892-903, 1998.
Ridgway,
S.H. and Benirschke, K., eds.: Breeding Dolphins; Present Status, Suggestions
for the Future. Report MNC 76/07, Marine Mammal Commission, Washington,
DC, November, 1977.
Ridgway,
S., Kamolnick, T., Reddy, M. and Curry, C.: Orphan-induced lactation in
Tursiops and analysis of collected milk. Marine Mamm. Sci. 11:172-182,
1995.
Robeck,
T.R.: Advances in the understanding and manipulation of bottlenose dolphin
reproduction. pp. 109-131, In, Bottlenose Dolphin Reproduction Workshop.
San Diego, California June 3-6, 1999. D. Duffield and T. Robeck, eds.
AZA Marine Mammal Taxon Advisory Group, Silver Spring, MD, 2000.
Rommel,
S.A., Pabst, D.A. and McLellan, W.A.: Functional morphology of the vascular
plexuses associated with the cetacean uterus. Anat. Rec. 237:538-546,
1993.
Sawyer-Steffan,
J.E. and Kirby, V.L.: A study of serum steroid hormone levels in captive
female bottlenose dolphins, their correlation with reproductive status,
and their application to ovulation induction in captivity. Natl. Techn.
Inf. Serv. PB 80-177199, 1980.
Sawyer-Steffan,
J.E., Kirby, V.L., and Gilmartin, W.C.: Progesterone and estrogens in
the pregnant and non-pregnant dolphin, Tursiops truncatus, and
the effects of induced ovulation. Biol. Reprod. 28:897-901, 1983.
Schroeder,
J.P. and Keller, K.V.: Seasonality of serum testosterone levels and sperm
density in Tursiops truncatus. J. Exp. Zool. 249:316-321, 1989.
Simpson,J.G.
and Gardner, M.B.: Comparative microscopic anatomy of selected marine
mammals. Chapter 5 in, Mammals of the Sea. Biology and Medicine. S.H.
Ridgway, ed. C.C. Thomas, Springfield, Illinois, pp. 298-418, 1972.
Slijper,
E.J.: Some remarks on gestation and birth in cetacea and other aquatic
mammals. Hvalradets Skrifter 41:1-62, 1956.
Slijper,
E.J.: Functional morphology of the reproductive system in Cetacea. Chapter
15 in, Whales, Dolphins, and Porpoises. K.S. Norris, ed. Univ. Calif.
Press, Berkeley, 1966.
Stump,
C.W., Robins, J.P. and Garde, M.L.: The development of the embryo and
membranes of the humpback whale, Megaptera nodosa (Bonaterre).
Austral. J. Marine and Freshw. Res. 11:365-386, 1960.
Turner,
W.: On the gravid uterus and on the arrangement of the foetal membranes
in the Cetacea (Orca gladiator). Trans. Roy. Soc. Edinburgh 26:467-504,
1872.
Walen,
K.H. and Madin, S.H.: Comparative chromosome analysis of the bottle-nosed
dolphin (Tursiops truncatus) and the pilot whale (Globicephala
scammonii). Am. Nat. 99:349, 1965.
Wells,
R.S.: Reproduction in wild bottlenose dolphins: Overview of patterns observed
during a long-term study. pp. 57-74, In, Bottlenose Dolphin Reproduction
Workshop. San Diego, California June 3-6, 1999. D. Duffield and T. Robeck,
eds. AZA Marine Mammal Taxon Advisory Group, Silver Spring, MD, 2000.
Wislocki
, G.B. and Enders, R.K.: The placentation of the bottle-nosed porpoise
(Tursiops truncatus). Amer. J. Anat. 68:97-114, 1941.
|