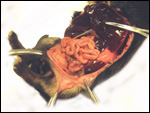
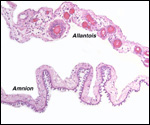
| |
9) Trophoblast external to barrier
Mossman (1987) said the following of specific giant cells at the floor of
the placenta in carnivora: "Most carefully examined carnivore placentas
have been shown to have scattered, usually moderately enlarged cells of
presumed maternal stromal origin alongside the maternal vessels of the zona
intima. These have been called "giant decidual cells" in the hyena
(Wynn & Amoroso, 1964) and cat (Malassiné, 1974), or simply "giant
cells" (Wynn & Björkman, 1968) in the cat and "decidual
cells" (Anderson, 1969) in the dog. Their ultrastructure in the dog
was described in detail by Anderson (1969) and in the cat by Malassiné
(1974). Obviously these cells of carnivores are not in the classical position
of decidua, but their presumed origin from endometrial stroma may justify
associating them tentatively with decidua. They are often not markedly large
(Anderson could not recognize them in paraffin sections of the dog placenta)
and have not been reported in raccoon, mustelids, or bears, possibly, as
Wimsatt (1974) has suggested, because specimen from these have not been
examined by electron microscopy. Both "giant" and "decidual"
cells must be considered tentatively designations until some definite anatomical
or physiological characteristics are discovered that set these cells distinctly
apart and justify a specific name."
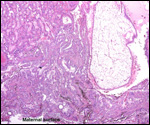
Extravillous packets of trophoblast do not exist and the invasion of endometrium
is very superficial, i.e., only through the superficial "compacta"
of the endometrium.
It is of historical interest to note that von Baer (1828) first convincingly
demonstrated that there was no confluence between the maternal and fetal
circulations. He injected a variety of uterine and fetal vessels of animals
with dyes and showed independence of the two vascular systems. This monograph
also provides a beautiful illustration of the dog placenta with partial
separation from the uterus and its broad green marginal regions. For historical
interest it might be nice to learn that this elegant contribution by v.
Baer was dedicated to S.T. v. Soemmerring at his 50th medical anniversary.
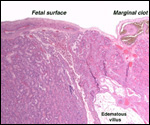
10) Endometrium
Dogs are said to have a "deciduate" placentation but typical decidual
cells are hard to identify, and endometrial glands are present throughout
pregnancy. Thus, no true decidua as in primates is found and there is further
disagreement whether all of the giant cells disappear after delivery of
the placenta.
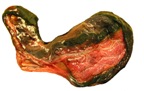
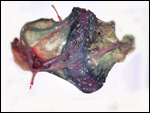
11) Various features
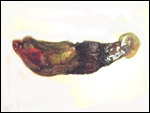
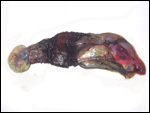
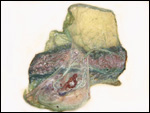
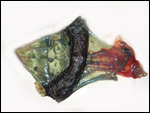
The marginal "hematoma" (green zone) has also been referred to
as "paraplacenta" or a hematophagous organ.
12) Endocrinology
The estrous cycle of dogs averages two per year. Ovulation is spontaneous.
Breeders suggest that the temperature of the pregnant bitch decreases
by about 1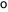 the
day before the delivery is expected. They infer from this that the temperature
drop relates to falling progesterone levels. Actual steroid measurements,
however, are unknown to us. Estrogen is not expected to be produced by
the placenta. Relaxin has apparently been demonstrated. Parturition is
believed to be induced by fetal cortisol and an increased prostaglandin
production by the gravid uterus. The canine corpus luteum is said to be
remarkably resistant to prostaglandin-induced regression. Prostaglandins
are also believed to cause the notorious vomiting in late pregnancy. the
day before the delivery is expected. They infer from this that the temperature
drop relates to falling progesterone levels. Actual steroid measurements,
however, are unknown to us. Estrogen is not expected to be produced by
the placenta. Relaxin has apparently been demonstrated. Parturition is
believed to be induced by fetal cortisol and an increased prostaglandin
production by the gravid uterus. The canine corpus luteum is said to be
remarkably resistant to prostaglandin-induced regression. Prostaglandins
are also believed to cause the notorious vomiting in late pregnancy.
The endocrine patterns of red wolves (Canis rufus) were delineated
in captive animals by fecal and serum analysis (Walker et al., 2002).
I realize that this is a different species, but it seems likely that the
results would be similar to what might be found in domestic dogs. These
investigators studied LH secretion and estrogens, as well as progesterone.
No native testosterone but a more polar androgen metabolite was detected
in males.
Oophorectomy or hypophysectomy during pregnancy leads to pregnancy termination
(Tienhoven, 1983). There are apparently no publications on the placental
production of gonadotropins or estrogens, although Courrier (1945) has
stated that no gonadotropins have been detected in the urine of the pregnant
dog. Histologically, the placenta does not give the impression of being
an endocrine organ.
The fetal/neonatal ovary has a large "interstitial gland".
13) Genetics
The domestic dog has 78 chromosomes. The autosomes are all acrocentrics;
the X chromosome is submetacentric and the Y is a diminutive metacentric
chromosome. Switonski et al. (1996) further defined the fine structure
of chromosomes with excellent G-banding. Hybridization has been reported
to occur with dingo, coyote, wolf, and possibly some foxes. Cats have
not hybridized with dogs, despite rumors in the popular press. A wide
variety of genetic diseases have been recorded in domestic dogs, best
known of which is perhaps the hip dysplasia in shepherds. I strongly recommend the review by Ostrander (2007) that describes in some detail the evolutionary genetic changes that have occurred in the evolution of dogs and its many races.
More recent information on genes and linkage maps are contained in a report
of a symposium on "Advances in Canine and Feline Genomics: Comparative
Genome Anatomy and Genetic Disease" in J. Hered. 94, Issue 1 (January),
2003. It contains a paper on 78XX/77C mosaics.
Several chromosomal errors have been identified in dog. Thus, Switonski
et al. (2000) found trisomy X in an infertile bitch. Her ovaries appeared
to be normal, but the dental arcades showed abnormalities. Sex-reversal
(male to female) was interpreted to be the result of a reciprocal X/A
translocation (Schelling et al., 2001). The bitch had ovotestes, uterus
and epididymis. Two infertile bitches were mosaic 78XX/77/X (Switonski
et al., 2003).
A comprehensive study of dog races, diseases, genetics, neoplasms and other topics has come from Ostrander et al. (2006).
 |
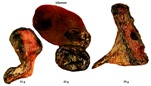
Karyotype of male and female domestic dogs. |
14)
Immunology
Despite the occasionally voiced notion that dogs have no blood group antigens,
the studies by Swisher et al. (1962) are a convincing demonstration of
their existence and of the development of iso-antibodies under appropriate
conditions.
The developmental landmarks of the immune system have recently been summarized by Holsapple et al. (2003). Splenic primordial appear on day 28, their “demarcation” occurs on day 45; thymic primordia are recognized on day 28 also, and hematopoiesis in the bone marrow begins on day 45. Proliferation in response to mitogens begins on day 50.
15)
Pathological features
Subinvolution of the placental site has occasionally been described (Beck
& McEntee, 1966). Their illustration suggests to me the retention of
placental tissue in the case that they described. Schlotthauer (1939) described
a choriocarcinoma in a 2-year-old bitch. The involution of the canine placental
site and of the adjacent endometrium has been reviewed in some detail by
McEntee (1990).
There is, of course, a vast literature on pathologic findings in dogs. They
can be accessed through texts on veterinary pathology and from the Armed
Forces Institute of Pathology (AFIP) in Washington.
16)
Physiological data
No data are known to me.
17) Other resources
Numerous dog-breeding clubs exist and can be identified locally. The Armed
Forces Institute of Pathology (AFIP) in Washington, DC has an extensive
repository of pathologic lesions from the guard dogs in the military services.
Cell lines of dog and related carnivores are available through CRES, the
research facility of the Zoological Society of San Diego.
18)
Future needs for investigation
Considering that so many placental abnormalities exist in human placentas,
one should expect that similar pathology might occur in other species.
With rare exception these have not been described. Future attention to
such pathologic lesions, especially in stillborn pups, might be rewarding.
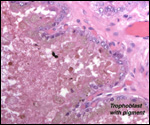
The green pigment should be better defined chemically.
References
Anderson,
J.W.: Ultrastructure of the placenta and fetal membranes of the dog. 1.
The placental labyrinth. Anat. Rec. 165:15-36, 1969.
Baer,
C.E.v.: Untersuchungen ueber die Gefaessverbindungen zwischen Mutter und
Frucht in den Saeugethieren. Leopold Voss, Leipzig, 1828.
Beck,
A.M. and McEntee, K.: Subinvolution of placental sites in a postpartum
bitch. A case report. The Cornell Vet. 56:269-277, 1966.
Björkman,
N.: Fine structure of the fetal-maternal area of exchange in the epitheliochorial
and endotheliochorial types of placentation. Acta anat. 86 (Suppl. 1): 1-22,
1973.
Cells
from the zoo's CRES organization via the Web: www.sandiegozoo.org.
Courrier,
R.: Endocrinologie de la Gestation. Paris, 1945.
Duval,
M.: Le placenta des carnassiers. J. Anat. Physiol. Paris 29:249-340,425-465,
663-729, 1893.
Gray, A.P.: Mammalian Hybrids. Commonwealth Agricultural Bureaux, Farnham
Royal, Slough, England, 1972.
Holsapple, M.P., West, L.J. and Landreth, K.S.: Species comparison of anatomical and functional immune system development. Birth Defect Res. B 68:321-334, 2003.
Kehrer,
A.: Zur Entwicklung und Ausbildung des Chorions der Placenta zonaria bei
Katze, Hund und Fuchs. Z. Anat. Entwickl.-Gesch. 143:25-42, 1973.
Malassiné,
A.: Evolution ultrastructurale du labyrinthe du placenta de chatte. Anat.
Embryol. 146:1-20, 1974.
McEntee,
K.: Reproductive Pathology of Domestic Mammals. Academic Press, San Diego,
1990.
Mossman,
H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills 1987.
Ostrander,
E.A., Giger, U. and Lindblad-Toh, K., eds: The Dog and its Genome. Cold Spring Harbor Press,
Cold Spring Harbor, New York, 2006.
Ramsey,
E.M.: The Placenta of Laboratory Animals and Man. Holt, Rinehart and Winston,
New York, 1975).
Schelling, C., Pienkowska, A., Arnold, S., Hauser, B. and Switonski, M.:
A male to female sex-reversed dog with a reciprocal translocation. J.
Reprod. Fertil. Suppl. 57:435-438, 2001.
Schlotthauer,
C.F.: Primary neoplasms in the genito-urinary system of dogs: a report
of ten cases. J. Am. Vet. Med. Ass. 95:181, 1939.
Selden,
J.R., Moorhead, P.S., Oehlert, M.L. and Patterson, D.F.: The Giemsa banding
pattern of the canine karyotype. Cytogenet. Cell Genet. 15:380, 1975.
Starck,
D.: Lehrbuch der speziellen Zoologie. Band II, Teil 5/2. Säugetiere.
Gustav Fischer, Jena, 1995.
Swisher,
S.N., Young, L.E. and Trabold, N.: In vitro and in vivo studies of the
behavior of canine erythrocyte-isoantibody systems. Ann. NY Acad. Sci.
97:15-25, 1962.
Switonski, M., Reimann, N., Bosma, A.A., Long, S., Bartnitzke, S., Pienkowska,
A., Moreno-Milan, M.M. and Fischer, P. (Committee for the Standardized
Karyotype of the Dog (Canis familiaris): Report of the progress
of standardization of the G-banded canine (Canis familiaris) karyotype.
Chromosome Research 4:306-309, 1996.
Switonski, M., Szczerbal, I., Grewling, J., Antosik, P., Nizanski, W.
and Yang, F.: Two cases of infertile bitches with 78,XX/77,X mosaic karyotypes:
a need for cytogenetic evaluation of dogs with reproductive disorders.
J. Hered. 94:65-68, 2003.
Switonski, M., Godynicki, S., Jackowiak, H., Pienkowska, A., Turczuk-Biertla,
I., Szymas, J., Golinski, P. and Bereszynski, A.: X trisomy in an infertile
bitch: cytogenetic, anatomic, and histologic studies. J. Hered. 91:149-150,
2000.
van
Tienhoven, A.: Reproductive Physiology of Vertebrates. Cornell Univ. Press,
Ithaca, 1983.
Vila,
C., Savolainen, P., Maldonado, J.E., Amorim, I.R., Rice, J.E., Honeycutt,
R., Crandall, K.A., Lundeberg, J. and Wayne, R.K.: Multiple and ancient
origins of the domestic dog. Science 276:1687-1679, 1997.
Walker, S.L., Waddell, W.T. and Goodrowe, K.L.: Reproductive endocrine
patterns in captive female and male red wolves (Canis rufus) assessed
by fecal and serum hormone analysis. Zoo Biol. 21:321-335, 2002.
Wimsatt,
W.A.: Morphogenesis of the fetal membranes and placenta of the black bear,
Ursus americanus (Pallas). Am. J. Anat. 140:471-495, 1974.
Wynn,
R.M. and Amoroso, E.C.: Placentation in the spotted hyena (Crocuta crocuta
Erxleben), with particular reference to the circulation. Am. J. Anat.
115:327-362, 1964.
Wynn,
R.M. and Björkman, N.: Ultrastructure of the feline placental membranes.
Am. J. Obstet. Gynecol. 102:34-43, 1968.
Wynn,
R.M. and Corbett, J.R.: Ultrastructure of the canine placenta and amnion.
Am. J. Obstet.Gynecol. 103:878-887, 1969.
|