| (Clicking
on the thumbnail images will launch a new window and a larger version
of the thumbnail.) |
| Last updated: Sept 15, 2007. |
Delacour’s Langur
Trachypithecus delacouri
Order: Primates
Family: Cercopithecidae (Colobinae)
1) General Zoological Data
Delacour’s langurs are black with white lateral cheeks and they have a strikingly crested hair streak on their heads; their newborns are golden-yellow. A reproductively active group of Delacour’s monkeys is housed at the EPRC Endangered Primate Rescue Station) in Cuc Phuong National Park, Vietnam, whence these placentas come. Nadler et al. (2007) estimated that this typical “limestone species” (because it is confined to limestone mountains) is severely endangered with only some 200-250 individuals left, living in 19 isolated populations. Poaching is the primary cause of decline but vanishing habitat is also considered.
The name Trachypithecus derives from the Greek, for ‘rough’, presumably because of their distribution of hair, while the revered Jean T. Delacour (1890-1985) provided the name Delacour, given to the species in his honor. Groves (2001) provided a detailed account of all those Asiatic leaf-eating monkeys and Nowak (1999) discussed the somewhat confused taxonomy further. He stated that T. delacouri is no longer considered to be a ‘good’ species but that it should be comprised under T. franciosi, a point discussed in some detail by Groves (2001) who grouped 6 primates as being of the “T. franciosi” group. Delacour’s monkey and one other species are restricted to Vietnam and a more detailed consideration of their distribution has recently been compiled by Groves (2007). Roos et al. (2007), on the other hand, have provided strong molecular evidence to suggest that T. delacouri should be considered as representing a ‘good species’. This web site has additional information on the placentation in the Francois’ monkey, Colobus monkey, and in Douc langurs that should be consulted for additional details.
 |
Delacour’s monkey pair and offspring at EPRC, Vietnam. |
.jpg) |
Limestone refuge at the protected Van Long Nature Reserve, Vietnam, a typical habitat for these animals. |
2) General Gestational Data
As is true for Francois’ langurs, there is virtually no published information on the cycle length which is estimated to be around 28 days. The gestational length is perhaps around 200+ days but it has not been accurately charted. Moreover, few other aspects of their gestational physiology are available. Singleton births are the rule.
3) Implantation
Early stages of implantation are not available for any of the Vietnamese langurs, but some studies have been published on a few samples of the related Dusky Leaf Monkey (Presbytis obscura). These were provided with some detail in Burton’s contribution of gravid uteri (1980). These specimens nicely demonstrated the superficial nature of implantation. Since the placenta is grossly similar to other cercopithecid species and because other similarities exist, it may be that the early implantational stages are quite similar to those of rhesus monkeys that have been treated recently in great detail by Enders (2007). The placentas are typically bidiscoid; only two single-lobed placentas have been observed, one in a Douc langur, the other in a Hatinh langur that weighed, however, 150 g and was very thick. The placenta implants relatively superficially in a uterus simplex (see Burton, 1980). Additional weights of langur placentas have been provided earlier (Benirschke et al., 2004) and data made available more recently do not differ appreciably from those measurements.
4) General Characterization of the Placenta
Two placentas of this species were available, the first one being depicted in 2004 and shown here again next (Benirschke et al., 2004). It weighed only 82 g and had a 21 cm cord. (More likely than not, however, the weight was ascertained by a poorly registering scale and may be incorrect, as the neonate thrived).
The second placenta also was a bidiscoid hemochorial organ and weighed 145 g. The disks differed slightly in size, with that disk with the cord insertion being slightly larger (9x7x2 cm vs. 6x5x2 cm in this case). Communicating vessels traverse from one lobe to the other within the chorionic membranes. Extravillous trophoblast infiltrates the decidua basalis and may also reach the superficial myometrium. Remarkably, there are no atrophying villi in the membranes as found in human placentas. Many placentas of cercopithecidae have small infarcts of placental tissue. Remarkably, none were found in these placentas.
5) Details of fetal/maternal barrier
This is a typically hemomonochorial placenta with a structure that is similar to that published in numerous other cercopithecidae. The term placenta has small villi, covered largely by syncytiotrophoblast; the underlying cytotrophoblast is no longer visible in sections but would be apparent by electronmicroscopy. Many villi have syncytial “bud” or “knots” that reach the intervillous space and are probably transported to the maternal lung. The villi contain few macrophages (‘Hofbauer cells’).
6) Umbilical cord
The cord has three vessels and may contain remnants of an atrophic allantoic duct. These cords were eccentrically inserted, 21 and 26 cm long, and had no significant spiraling. Their surfaces were covered with a thin amnion epithelium.
.jpg) |
Umbilical cord with vein at top right. |
.jpg) |
Section of umbilical cord with remnant of discontinuous allantoic duct at right. |
7) Uteroplacental circulation
The maternal decidual arterioles are infiltrated and their walls are partly replaced by extravillous trophoblast, as is shown in subsequent photomicrographs. I have not seen the so-called ‘atherosis’ of pre-eclampsia in any of these placenta, irrespective of whether the placentas contained infarcts.
8) Extraplacental membranes
The amnion is avascular, has a single flat epithelial surface and dense connective tissue layer that adheres to the underlying chorion. This contains the fetal vessels although only few large vessels span from one lobe to the other. Beneath the chorion lies a variably thick layer of extravillous trophoblast, but atrophied villi are not found although they are abundant in human membranes. The decidua capsularis peripherally is not exceptional.
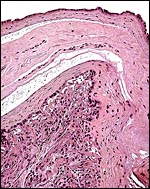 |
Amnion on top, loosely attached to the chorion that is underlain by a single layer of extravillous trophoblast and then follows the ‘decidua capsularis’. |
9) Trophoblast external to barrier
There is probably infiltration of the myometrium with extravillous trophoblast (“X-cells”), as is the rule in many other primates with hemochorial placentation.
10) Endometrium
Normal decidual transformation is present, similar to that found in most other higher primate placentas. Some extravillous trophoblast is found in between the decidual stromal cells and vessels are altered by this trophoblast. Selenka (1903) has described in some detail the extensive decidual reaction of langur implantation.
.jpg) |
Decidua basalis with maternal arterioles. |
11) Various features
None are known to me in this species.
12) Endocrinology
No endocrine studies have been done in this species; but the few endocrine studies that have been done in Douc langurs are summarized in that chapter and were presented by Czekala et al. in 1981. They also calculated the menstrual cycle of one Douc langur. More recently, Ademmer et al. (2002) have determined the length of cycles with steroid analysis of feces in three captive groups of douc langurs at the Cologne zoo. They found an average cycle length of 28 days with the follicular phase lasting 13 days, and the luteal phase was 11 days long. No such data exist for Delacour’s langur.
13) Genetics
Virtually all leaf-eating monkeys, and certainly this species, have 44 very similar-appearing chromosomes (Benirschke et al., 2004). Roos et al. (2007) studied the mitochondrial cytochrome b gene variation in 123 individual Trachypithecus leaf monkeys and suggested that this animal represents indeed a separate species.
Hybrids have not been described.
.jpg) |
Karyotype of Delacour’s monkey, originating from EPRC, Vietnam. |
14) Immunology
There are no such studies.
15) Pathological features
Placental infarcts are common and occasional retroplacental hematomas occur. Infection with Helicobacter is also common. More detailed pathological studies on Delacour’s monkeys are no available, but Griner (1983) summarizes much experience with many other leaf-eating monkeys’ mortality. Various infections (including pulmonary acariasis) and gastric problems are prominently mentioned.
16) Physiologic data
The locomotor behavior was explored in detailed observations by Workman & Covert (2005). Robinson (2004) has provided some details of keeping langurs in captivity that are useful. The data also include diets, housing and mortalities. There is also the extensive consideration of the food intake and folivory in the important publication by Ademmer et al (2002) that addresses the need for long periods of sleep in this species and the susceptibility to stress. Even more important are the observations by Bauchop & Martucci (1968) that first described the ruminant-like digestion of the portioned stomach by these leaf-eating monkeys.
17) Other resources
Cell strains of fibroblasts are kept at CRES of the Zoological Society of San Diego and can be obtained by contacting Dr. Oliver Ryder (oryder@ucsd.edu).
 |
This wonderful group of stamps of Vietnamese primates was issued in 2002. |
18) Other remarks – What additional Information is needed?
Early stages of implantation, similar to those by Burton (1980), are highly desirable, as are endocrine data.
Acknowledgement
The animal photograph in this chapter and the placentas come from Tilo Nadler at EPRC, Cuc Phuong National Park, Vietnam.
References
Ademmer, C., Klumpe, K., v.Maravic, I., Königshofen and Schwitzer, C.: Nahrungsaufnahme und Hormonstatus von Kleideraffen (Pygathrix n. nemaeus Linnaeus, 1771) im Zoo. Z. Kölner Zoo 45:129-135, 2002.
Bauchop, T. and Martucci, R.W.: Ruminant-like digestion of the langur monkey. Science 161:698-700, 1968.
Benirschke, K., Houck, M., Streicher, U. and Nadler, T.: Cytogenetic aspects of langurs, Chapter 7 in: Nadler, T, Streicher, U. and Ha Thang Long: Conservation of Primates in Vietnam. Haki Publishing, Vietnam and Frankfurt Zoological Society, pp. 37-40, 2004.
Benirschke, K., Streicher, U and Nadler, T.: The placenta of leaf monkeys, Chapter 24 in: Nadler, T, Streicher, U. and Ha Thang Long: Conservation of Primates in Vietnam. Haki Publishing, Vietnam and Frankfurt Zoological Society, pp. 144-146, 2004.
Burton, G.J.: Early placentation in the dusky leaf monkey (Presbytis obscura). Placenta 1:187-195, 1980.
Czekala, N.M., Hodges, J.K. and Lasley, B.L.: Pregnancy monitoring in diverse primate species by estrogen and bioactive luteinizing hormone determinations in small volumes of urine. J. Med. Primatol. 10:1-15, 1981.
Enders, A.C.: Implantation in the macaque: Expansion of the implantation site during the first week of implantation. Placenta 28:794-802, 2007.
Griner, L.A.: Pathology of Zoo Animals. Zool. Soc. San Diego, pp. 366-367, 1983.
Groves, C.: Primate Taxonomy. Smithsonian Institution, Washington, DC, 2001.
Groves, C.: Speciation and biogeography of Vietnam’s primates. Vietnamese J. Primatol. 1:27-40, 2007.
Nadler, T.: Distribution and status of the Delacour’s langur (Trachypithecus delacouri) and recommendations for its long-term conservation, Chapter 11 in: Nadler, T, Streicher, U. and Ha Thang Long: Conservation of Primates in Vietnam. Haki Publishing, Vietnam and Frankfurt Zoological Society, pp. 63-71, 2004.
Nadler, T., Streicher, U. and Ha Thang Long: Conservation of Primates in Vietnam. Haki Publishing, Vietnam and Frankfurt Zoological Society, 2004.
Nadler, T., Vu Ngoc Thanh and Streicher, U.: Conservation status of Vietnamese primates. Vietnamese J. Primatol. 1:7-26, 2007.
Nowak, R.M.: Walker’s Mammals of the World. 6th ed. The Johns Hopkins Press, Baltimore, 1999.
Robinson, R.: Breeding and management of Francois's langurs at Belfast zoo. Int. Zoo News 51:200-208, 2004.
Roos, C., Vu Ngoc Thanh, Walker, L. and Nadler, T.: Molecular systematics of Indochinese primates. Vietnamese J. Primatol. 1:41-53, 2007.
Selenka, E.: Zur vergleichenden Keimesgeschichte der Primaten. Studien über Entwicklungsgeschichte der Tiere. Heft 10. Menschenaffen, L. 5, pp. 329-372, F. Keibel, Wiesbaden, 1903.
Workman, C. and Covert, H.H.: Learning the ropes: The ontogeny of locomotion in red-shanked douc (Pygathrix nemaeus), Delacour’s (Trachypithecus delacouri), and Hatinh langurs (Trachypithecus hatinhensis). I. Positional behavior. Amer. J. Phys. Anthropol. 128:371-380, 2005.

.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)