| |
The chromosome number of cervidae is variable. The usually high chromosomal
number in many deer species (around 2n=70) presumably reflects their closer
relationships, while a few "outliers" are also somewhat removed
from the mainstream of cervidae (see Yang et al, 1997). We have established
the following chromosome numbers: Moose (70); Axis deer - chital (66); Hog
deer (68); Roe deer (70); Barasingha deer (56); Red deer (68); Sambar deer
60; 64-65); Fallow deer - Dama (68); Père David's deer (68); Chinese
water deer (Hydropotes inernis) (70); Red brocket deer (Mazama)
(50); Reindeer (70); Pudu (70); White-tailed deer (70); Mule deer (70);
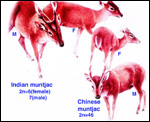
Chinese muntjac (46); Indian muntjac (6/7) [see Hsu & Benirschke]. Since
then, numerous chromosomal banding studies have been done on some of these
species and additional species have also been studied: Tufted deer (Elaphodus
cephalophus) 2n=46-48 (Shi et al., 1991); white-lipped deer (Cervus
albirostris Przewalski) 2n=66 (Wang et al., 1982), and several other muntjacs.
Eld's deer (Cervus eldi) has 2n=58 (Neitzel, 1979, she also lists
another group of deer species). Sika deer (Cervus nippon hortulorum Swinhoe)
has 2n=64-68 (Gustavsson & Sundt, 1969). Another complete listing may
be found in Groves & Grubb (1987).
Evolutionary relationships have been discussed at the beginning of this
chapter. Modern studies are beginning to provide further insight into disputed
areas. Thus, the mtDNA of muntjacs was explored by Lan & Shi (1993).
Repetitive DNA of cervids was used for evolutionary questions by Bogenberger
et al. (1987). Chromosome "painting" led to a better understanding
of muntjac chromosomal fusions (Yang et al., 1995). mtDNA was used in studying
hybrids between mule and white-tailed deer (Carr et al, 1986), and so forth.
Genetic heterozygosity positively influenced fetal size and growth in white-tailed
deer, while number of fetuses correlated negatively (Cothran et al., 1983).
14) Immunology
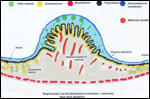
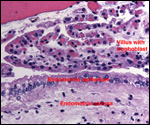
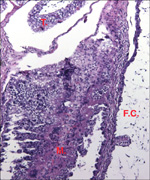
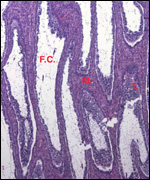
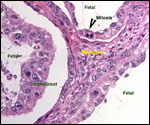
Lymphocytic infiltration of the uterine mucosa/epithelium is a frequent,
if not regular, feature of ruminant uteri. Lee et al. (1995) studied this
in six species of deer. They found a significant cellular increase to occur
from early implantation to mid-gestation. They observed also that the size
of granules of these cells varies greatly and that they increase with gestation.
The investigators observed that these lymphocytes tend to be close to regions
where the trophoblastic binucleated cells fuse with the endometrial epithelium.
Here they produce the trinucleate cells and give up their granules. The
precise reason for these changes remains unknown but "immune recognition" is suspected..
15)
Pathological features
Fetal demise of one of twins has been described in an elk uterus (Saunders,
1955), and Mansell & Cringan (1968) found two dead female fetuses
with a live female triplet in the uterus of a white-tailed deer. The dead
fetuses were enclosed in a single chorion (? monozygotic) and their cotyledons
had detached. They referred to other cases of this fetal absorption and
attributed it to poor nutrition. We have seen this in axis deer and found
no good explanation for the demise.
Major die-offs can occur in island deer due to overcrowding. This was
described for sika deer by Christian et al. (1960). In Texas, abnormal
antler growth of white-tailed deer was the result of hypogonadism, presumed
to result from some poison (Taylor et al, 1964).
Numerous infections have been described in a variety of deer: Toxoplasmosis
in mule deer (Dubey, 1982); Theileria cervi infection in white-tailed
deer (Schaeffler, 1963); Pneumostrongylus tenuis infection of white-tailed
deer (Anderson, 1965), and others. Most important at present is the transmission
of the spirochetal organism Borrelia burgdorferi that causes Lyme
disease and is transmitted from wild mice to man by the deer tick Amblyomma
americanum (e.g. Ebel et al., 2000).
Ratcliffe (1968) reported abnormal antler growth and "wasting" in captive white-tailed deer and related it to abnormal pituitary/adrenal
activity. Trophoblastic tumors have not been described, nor are ascending
intrauterine infections features of cervid gestations.
The San Diego Zoo has traditionally held many cervid species and, consequently,
Griner (1983) gathered much pathological material. Most deaths he recorded
were due to trauma, anesthesia, fracture, fights and old age. Dental disease
was not uncommon in captivity and outbreaks of malignant catarrhal fever
and of blue tongue virus infection were recorded. Only two congenital
heart diseases, one cleft palate and one congenital goiter were observed
as anomalies, and very rare neoplasms (one lymphosarcoma; a biliary carcinoma
in an axis deer, even though deer have no gall bladder) were found. Some
parasites posed a continued problem, but reproductive pathology occurred
rarely.
16) Physiological data
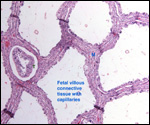
The placenta of some deer species produces a pregnancy-specific protein
B PSPB) which Huang and colleagues (1999) found to be similar, in preparations
from moose and elk, to that extracted from cow and sheep placentas. The
growth of mule deer fetuses and embryos was methodically recorded by Hudson & Browman (1959). Serum protein profiles of white-tailed and mule
deer were studied by Cowan & Johnston (1962). They found great similarity
in quantities, but different electrophoretic mobility in these sympatric Odocoileus species.
I am not aware of any studies of blood flow, blood volume, or blood pressures
records. It is well known, however, that many deer species have a physiologic
tendency to develop sickled erythrocytes. In contrast to human sickle
cell anemia patients whose sickling occurs in oxygen-poor environments,
that of deer species is initiated by increased oxygenation. The sickle
cells are very similar to human sickled red blood cells, with tactoids
and similar shapes, but there is no disease associated with deer sickling.
It is due to hemoglobin polymorphism. (Undritz et al., 1960; Pritchard
et al., 1963; Naik et al., 1964; Kitchen et al., 1964).
17)
Other resources
For a wide variety of cervid species the "Frozen Zoo" of CRES
at San Diego Zoo possesses fibroblast cell lines and most have had chromosomal
analysis.
18) Other relevant features and information needed in future
Several species of deer have become farm animals, especially so with the
advent of a complete understanding of their reproductive physiology. Thus,
Fletcher (2001) gives examples of farming of Cervus elaphus, C.
e. canadensis, Dama dama and D. d. mesopotamica, with
successful embryo transfer and other artificial breeding techniques. We
know too little of the endocrinology and cord lengths. Also, do placental
abnormalities occur?
References
Aitken, R.J.: Ultrastructure of the blastocyst and endometrium of the
roe deer (Capreolus capreolus) during delayed implantation. J.
Anat. 119:369-384, 1975.
Aitken,
R.J., Burton, J., Hawkins, J., Kerr-Wilson, R., Short, R.V. and Steven,
D.H.: Histological and ultrastructural changes in the blastocyst and reproductive
tract of the roe deer, Capreolus capreolus, during delayed implantation.
J. Reprod. Fertile. 34:481-493, 1973.
Anderson,
R.C.: The development of Pneumostrongylus tenuis in the central
nervous system of white-tailed deer. Path. Vet. 2:360-379, 1965.
Bogenberger,
J.M., Neitzel, H. and Fittler, F.: A highly repetitive DNA component common
to all Cervidae: its organization and chromosomal distribution during
evolution. Chromosoma 95:154-161, 1987.
Carr,
S.M., Ballinger, S.W., Derr, J.N., Blankenship, L.H. and Bickham, J.W.:
Mitochondrial DNA analysis of hybridization between sympatric white-tailed
deer and mule deer in west Texas. Proc. Natl. Acad. Sci. USA 83:9576-9580,
1986.
Cell
lines are available from CRES at the San Diego Zoo under: www.sandiegozoo.org
Chapman,
D.I. and Dansie, O.: Unilateral implantation in the muntjac deer. J. Zool.
Lond. 159:534-536, 1969.
Christian,
J.J., Flyger, V. and Davis, D.E.: Factors in the mass mortality of a herd
of sika deer, Cervus nippon. Chesapeake Sci. 1:79-95, 1960.
Cothran,
E.G., Chesser, R.K., Smith, M.H. and Johns, P.E.: Influences of genetic
variability and maternal factors on fetal growth in white-tailed deer.
Evolution 37:282-291, 1983.
Cowan,
I.M. and Johnston, P.A.: Blood serum protein variations at the species
and subspecies level in deer of the genus Odocoileus. Systematic
Zool. 11:131-138, 1962.
Dubey,
J.P.: Isolation of encysted Toxoplasma gondii from muscles of mule
deer in Montana. JAMA 181:1535, 1982.
Ebel,
G.D., Campbell, E.N., Goethert, H.K., Spielman, A. and Telford, S.R.:
Enzootic transmission of deer tick virus in New England and Wisconsin
sites. Amer. J. Trop. Med. Hyg. 63:36-42, 2000.
Fletcher,
T.J.: Deer: New domestic farm animals defined by controlled breeding.
In press, 2001.
Gadsby,
J.E., Heap, R.B. and Burton, R.D.: Oestrogen production by blastocyst
and early embryonic tissue of various species. J. Reprod. Fertil. 60:409-417,
1980.
Gray,
A.P.: Mammalian Hybrids. Second edition. A Check-List with Bibliography.
Commonwealth Agricultural Bureaux, Farnham Royal, Slough, UK, 1972.
Griner,
L.A.: Pathology of Zoo Animals. Zoological Society of San Diego, 1983.
Groves,
C.P. and Grubb, P.: Relationships of living deer. In, Biology and Management
of the Cervidae. C.M. Wemmer, ed. Smithsonian Inst. Press, Washington,
1987, pp. 21-59.
Gustavsson, I. And Sundt, C.O.: Three polymorphic chromosome systems of
centric fusion type in a population of Manchurian Sika deer (Cervus nippon
hortulorum Swinhoe). Chromosoma 28:245-254, 1969.
Hamilton,
W.J., Harrison, R.J. and Young, B.A.: Aspects of placentation in certain
Cervidae. J. Anatomy 94:1-33, 1960.
Harrison,
R.J. and Hamilton, W.J.: The reproductive tract and the placenta and membranes
of Père David's deer (Elaphurus davidianus Milne Edwards).
J. Anat. 86:203-224, 1952.
Hsu,
T.C. and Benirschke, K.: An Atlas of Mammalian Chromosomes. Springer-Verlag,
New York, 1975.
Huang,
F., Cockrell, D.C., Stephenson, T.R., Noyes, J.H. and Sasser, R.G.: Isolation,
purification, and characterization of pregnancy-specific protein B from
elk and moose placenta. Biol. Reprod. 61:1056-1061, 1999.
Hudson,
P. and Browman, L.G.: Embryonic and fetal development of the mule deer.
J. Wildlife Manag. 23:295-304, 1959.
Kitchen,
H., Putnam, F.W. and Taylor, W.J.: Hemoglobin polymorphism: Its relation
to sickling of erythrocytes in white-tailed deer. Science 144:1237-1239,
1964.
Kurnosov,
K.M.: Interfetal placental connections of the elk in embryonic parabiosis.
Proc. Akad. Nauk. SSR Doklady, Biol. Sect. 142:92-94, 1962 (in English).
Lan,
H. and Shi, L.: Restricted endonuclease analysis of mitochondrial DNA
of muntjac and related deer. In, Deer of China, N. Ohtaishi and H.-I.
Sheng, eds. Elsevier Science Publ., 1993.
Lawn,
A.M., Chiquoine, A.D. and Amoroso, E.C.: The development of the placenta
in the sheep and goat: an electronmicroscope study. J. Anat. 105:557-578,
1969.
Lee,
C.S., Gogolin-Ewens, K. and Brandon, M.R.: Comparative studies on the
distribution of binucleate cells in the placentae of the deer and cow
using monoclonal antibody, SBU-3. J. Anat. 147:163-179, 1986.
Lee,
C.S., Wooding, F.B. and Morgan, G.: Quantitative analysis of intraepithelial
large granular lymphocyte distribution and maternofetal cellular interactions
in the synepithelichorial placenta of the deer. J. Anat. 187:445-460,
1995.
McMahon,
C.D., Fisher, M.W., Mockett, B.G. and Littlejohn, R.P.: Embryo development
and placentome formation during early pregnancy in red deer. Reprod. Fertil.
Dev. 9:723-730, 1997.
Mansell,
W.D. and Cringan, A.T.: A further instance of fetal atrophy in white-tailed
deer. Canad. J. Zool. 46:33-34, 1968.
Miyamoto,
M.M., Kraus, F. and Ryder, O.A.: Phylogeny and evolution of antlered deer
determined from mitochondrial DNA sequences. Proc. Natl. Acad. Sci. USA
87:6127-6131, 1990.
Mossman,
H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Naik,
S.N., Bhatia, H.M., Baxi, A.J. and Naik, P.V.: Hematological study of
Indian spotted deer (Axis deer). J. Exper. Zool. 155:231-236, 1964.
Neitzel,
H.: Chromosomenevolution in der Familie der Hirsche (Cervidae).
Bongo 3:27-38, 1979.
Nowak,
R.M. and Paradiso, J.L.: Walker's Mammals of the World, Vol. II. 4th edition.
The Johns Hopkins University Press, Baltimore, 1983.
Plotka,
E.D.: Deer. In, Encyclopedia of Reproduction. E. Knobil and J.D. Neill,
eds. Academic Press, San Diego 1998, Vol. I, pp.842-857.
Pritchard,
W.R., Malewitz, T.D. and Kitchen, H.: Studies on the mechanism of sickling
of deer erythrocytes. Exp. Molec. Pathol. 2:173-182, 1963.
Puschmann,
W.: Zootierhaltung. Vol. 2, Säugetiere. VEB Deutscher Landwirtschaftsverlag,
Berlin, 1989.
Randi,
E., Mucci, N., Claro-Hergueta, F., Bonnet, A. and Douzery, E.J.P.: A mitochondrial
DNA control region phylogeny of the Cervinae: speciation in Cervus and
implications for conservation. Animal Conserv. 4:1-11, 2001.
Ratcliffe:
Environment, behavior and disease: Observations and experiments at the
Philadelphia Zoological Garden. Trans. & Studies Coll. Physic. Philadelphia
36:7-21, 1968.
Saunders,
J.K.: Fetus in yearling cow elk, Cervus canadensis. J. Mammal.
36:145, 1955.
Schaeffler,
W.F.: Serologic tests for Theileria cervi in white-tailed deer
and for other species of Theileria in cattle and sheep. Amer. J.
Vet. Res. 24:784-791, 1963.
Shi,
L., Ye, Y., Duax, X.: Comparative cytogenetic studies on the red muntjac,
Chinese muntjac, and their F1 hybrids. Cytogenet. Cell Genet. 26:22-27,
1980.
Shi,
L. and Pathak, S.: Gametogenesis in a male Indian muntjac x Chinese muntjac
hybrid. Cytogenet. Cell Genet. 30:152-156, 1981.
Shi,
L., Yang, F. and Kumamoto, A.: The chromosomes of tufted deer (Elaphodus
cephalophus) Cytogenet. Cell. Genet. 56:189-192, 1991.
Sinha,
A.A., Seal, U.S., Erickson, A.W. and Mossman, H.W.: Morphogenesis of the
fetal membranes of the white-tailed deer. Amer. J. Anat. 126:201-242,
1969.
Sinha,
A.A., Seal, U.S., Erickson, A.W.: Ultrastructure of the amnion and amniotic
plaques of the white-tailed deer. Amer. J. Anat. 127:369-396, 1970.
Taylor,
D.O.N., Thomas, J.W. and Marburger, R.G.: Abnormal antler growth associated
with hypogonadism in white-tailed deer in Texas. Amer. J. Vet. Res. 25:179-185,
1964.
Undritz,
E., Betke, K. and Lehmann, H.: Sickling phenomenon in deer. Nature 187:333-334,
1960.
Vrba,
E.S. and Schaller, G.B., eds.: Antelopes, Deer, and Relatives. Fossil
Record, Behavioral Ecology, Systematics, and Conservation. Yale University
Press, New Haven, 2000.
Wang,
Z., Du, D.R., Xu, J. and Che, Q.: Karyotype, C-banding and G-banding patterns
of white-lipped deer (Cervus albirostris Przewalski). Acta Zool.
Sin. 28:250-255, 1982.(in Chinese).
Weldon,
W.F.R.: Note on placentation of Tetraceros quadricornis. Proc.
Zool. Soc. London pp.2-4, 1884. (Cited by Mossman, 1987).
Wooding,
F.B.: The role of the binucleate cell in ruminant placental structure.
J. Reprod. Fertil. Suppl. 31:31-39, 1982.
Wooding,
F.B.: Frequency and localization of binucleate cells in the placentomes
of ruminants. Placenta 4:527-539, 1983.
Wooding,
F.B., Morgan, G. and Adam, C.L.: Structure and function in the ruminant
synepitheliochorial placenta: central role of the trophoblast binucleate
cell in deer. Microsc. Res. Tech. 38:88-99, 1997.
Wurster,
D.H. and Benirschke, K.: Chromosome studies in some deer, the springbok,
and the pronghorn, with notes on placentation in deer. Cytologia 32:273-285,
1967.
Yang, F., Carter, N.P., Shi, L. and Ferguson-Smith, M.A.: A comparative
study of karyotypes of muntjacs by chromosome painting. Chromosoma 103:642-652,
1995.
Yang,
E., O'Brien, P.C.M., Wienberg, J., Neitzel, H., Kin, C.C. and Ferguson-Smith,
M.A.: Chromosomal evolution of the Chinese muntjac (Muntjacus reevesi).
Chromosoma 106:37-43, 1997.
|