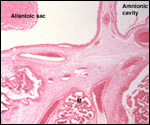
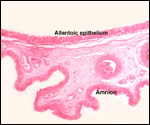
| |
The
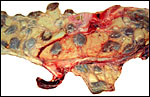
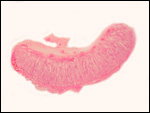
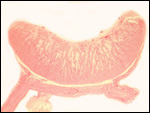
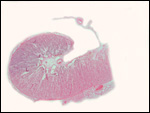
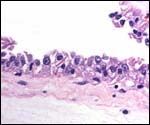
intercotyledonary membranes and their relation to the uterus have been
best studied by Davies & Wimsatt (1966) who defined the regions of
the so-called "areolae". These areas are located atop the orifices
of endometrial glands and are presumed to be an important zone of nutritional
transfer to the fetus. This is the site of "uterine milk" production
by the endometrium. The remainder shows a close approximation of the microvillous
surfaces of endometrial epithelium and cellular trophoblast. There is
no decidua capsularis.
9)
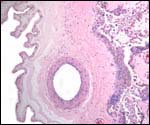
Trophoblast external to barrier
There is no extravillous trophoblastic invasion.
10)
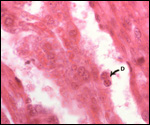
Endometrium
There is no decidualization.
11)
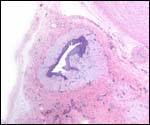
Various features
There is no subplacenta.
12)
Endocrinology
The reproductive physiology and nutritional influences on reproduction
of does have been superbly summarized by Keisler (1998). The length of
the estrous cycle is about 19-21 days; offspring are born usually only
yearly. PGF2", produced by the uterus, lyses the corpus luteum; this
consequently leads to a fall of progesterone. When pregnancy is established,
the placenta produces PSPB (Pregnancy Specific Protein B), whose function
is unknown; placental lactogen and other products are also secreted. PSPB
is used for pregnancy testing. After 60 days of gestation, the placental
progesterone production is adequate enough to support pregnancy. Later
in pregnancy, the adrenal/pituitary axis takes over the endocrine support,
and delivery can be induced by injection of adrenal steroids (cortisol).
Conversely, hypophysectomy (in sheep fetuses) or specific cranial anomalies
(anencephaly), lead to postmaturity. Later in gestation, PGE2 is produced.
13)
Genetics
Goats have 60 chromosomes, as do the many specimens of Cretan goats studied
at CRES of the Zoological Society of San Diego.
Studies of mtDNA by Luikart et al. (2991) found divergence of goat and
cattle to have occurred about 200,000 years ago, while goat domestication
is estimated as having taken place about 10,000 years ago, but originating
from three independent lines of ancestors.
It has occasionally been possible to hybridize sheep and goats, and an
extensive literature exists about these attempts (Gray, 1972). Most fetuses
abort by two months; their chromosome number is 57, in between the sheep
(54) and that of the goat (60). In a large series of experiments, Dent
et al. (1971) showed with electronmicroscopy that the trophoblast of the
hybrids is normal and implantation appears to proceed normally. There
was, however, an extensive accumulation of platelets in the maternal blood
vessels and endothelial swelling to the point of occlusion with hemorrhage
ensuing between 34 and 38 days. These authors suggested that this is akin
to an immunological rejection of the fetal hybrid implant. Domestic goat
and Cretan goats "hybridize" with fertile offspring. Occasional
female sheep x goat hybrids have been fertile (Bunch et al. (1976), when
bred back to Barbados sheep, resulting in animals with 55 chromosomes.
14)
Immunology
MHC molecules, NK cells, and other specific cell populations have apparently
not been studied much in goats. Hemolysins were found in sheep x goat
hybrid pregnancies but were not considered to have caused the abortions
(Tucker et al., 1971). Immunization of goats against sheep antigens reduced
the length of fetal survival from six to three weeks (McGovern, 1973).
15)
Pathological features
The findings at autopsy of adults have shown that Cretan goat deaths were
usually due to trauma (aggression) and also attributed to old age with
blindness; only occasional infections have occurred. Fetal or neonatal
deaths have been attributed to poor nursing of twins, "stress",
and occasional congenital anomalies (renal agenesis). Infections are uncommon,
except for males who may suffer Brucella epididymitis with sterility ensuing.
This organism also causes abortion by infecting the placenta. Anderson
& Cheville (1986) showed large numbers of organisms in these placentas
within the rough endoplasmic reticulum of the cytotrophoblast but absent
from the binucleated cells.
16)
Physiological data
Physiological parameters have been extensively explored, but mostly in
sheep, and the reader should refer to that chapter for details and references.
Fetal carbohydrate availability consists most of fructose, as opposed
to glucose of other species.
17)
Other resources
The San Diego Zoo has a thriving colony of these animals from which animals
cell cultures are available through CRES.
18)
Other relevant features and additional needs for information
More knowledge of the length of umbilical cords would be desirable and
whether pathologic features can be identified. The precise mechanism leading
to the abortion of sheep x goat hybrids should be better understood.
References
Amoroso, E.C.: Placentation. In, Marshalls Physiology of Reproduction.
3rd ed. A.S. Parkes, ed. Longmans, London. 1952. Chapter 15, pp. 127-311.
Anderson,
T.D. and Cheville, N.F.: Ultrastructural morphometric analysis of Brucella
abortus-infected trophoblasts in experimental placentitis. Amer. J. Pathol.
124:226-237, 1986.
Bautzmann,
H. and Schröder, R.: Vergleichende Studien über Bau und Funktion
des Amnions. Z. Zellforsch. 43:48-63, 1955.
Bunch,
T.D., Foote, W.C. and Spillett, J.J.: Sheep-goat hybrid karyotypes. Theriogenology
6:379-385, 1976.
Cell
cultures are available from CRES at: www.sandiegozoo.org/CRES
Davies,
J. and Wimsatt, W.A.: Observations on the fine structure of the sheep
placenta. Acta anat. 65:182-223, 1966.
Dent,
J., McGovern, P.T. and Hancock, J.L.: Immunological implications of ultrastructural
studies of goat x sheep hybrid placentae. Nature 231:115-117, 1971.
Gray,
A.P.: Mammalian Hybrids. Second edition. A Check-List with Bibliography.
Commonwealth Agricultural Bureaux, Farnham Royal, Slough, UK, 1972.
Kaufmann,
P.: Vergleichend-anatomische und funktionelle Aspekte des Placenta-Baues.
Funkt. Biol. Med. 2:71-79, 1983.
Keisler,
D.H.: Sheep and Goats. In, Encyclopedia of Reproduction, Vol. 4, E. Knobil
and J.D. Neill, eds. Academic Press, San Diego, pp. 479-492.
King,
G.J., Atkinson, B.A. and Robertson, H.A.: Implantation and early placentation
in domestic ungulates. J. Reprod. Fertil. Suppl. 31:17-30, 1982.
Naaktgeboren,
C. and Zwillenberg, H.H.L.: Untersuchungen über die Auswüchse
und an der Nabelschnur bei Walen und Huftieren, mit besonderer Berücksichtigung
des europäischen Hausrindes. Acta Neerl.-Scand. 4:31-60, 1961.
Luikart,
G., Gielly, L., Excoffier, L., Vigne, J.D., Bouvet, J. and Taberlet, P.:
Multiple maternal origins and weak phylogeographic structure of goats.
Proc, Natl. Acad. Sci. USA 98:5927-5932, 2001.
Makowski,
E.L.: Maternal and fetal vascular nets in placentas of sheep and goats.
Amer. J. Obstet. Gynecol. 100:283-288, 1068.
McGovern,
P.T.: The effect of maternal immunity on the survival of sheep x goat
hybrid embryos. J. Reprod. Fertil. 34:215-220, 1971.
Mossman,
H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Nowak,
R.M.: Walker's Mammals of the World, Vol. II. The Johns Hopkins University
Press, Baltimore, 1999.
Ross,
M.G., Ervin, M.G., Rappaport, V.J., Youssef, A., Leake, R.D. and Fisher,
D.A.: Ovine fetal urine contribution to amniotic and allantoic compartments.
Biol. Neonat. 53:98-104, 1988.
Tucker,
E.M., McGovern, P.T. and Hancock, J.L.: Serological investigations into
the cause of death of goat x sheep hybrid fetuses. J. Reprod. Fertil.
27:417-425, 1971.
Wimsatt,
W.A.: New histological observations on the placenta of the sheep. Amer.
J. Anat. 87:391-458, 1950.
Wooding,
F.B.P.: Role of binucleate cells in fetomaternal cell fusion at implantation
in the sheep. Amer. J. Anat. 170:233-250, 1950.
Wooding,
F.B.P., Flint, A.P.F., Heap, R.B. and Hobbs, T.: Autoradiographic evidence
for migration and fusion of cells in the sheep placenta: Resolution of
a problem in placenta classification. Cell Biol. Internat. Reports 5:821-827,
1981.
|