|
(Clicking
on the thumbnail images below will launch a new window and a larger
version of the thumbnail.)
|
| Last updated: July 23, 2005. |
Domestic
Cattle
Bos taurus
Order: Artiodactyla
Family: Bovidae
1) General Zoological Data
The earliest known species of Bos (and including Bibos) is Bos
acutifrons "from the lowest Pleistocene (+/- 1.8 MYA) of the
Siwalik Hills in Nothern India" (Pilgrim, 1947). While it is often
assumed that Bos is the earliest form of this line, the studies by Groves
(1981) show pretty conclusively that the ascendancy is more like this:
gaur, banteng, bison, yak, kouprey and aurochs. Later in the Pleistocene
the most primitive of direct bovine ancestors spread widely over Europe
and Africa and formed new species, such as the enormous Aurochs that died
out in the 17th century. Oxens had split off the bovid lines that led
to Bubalus and Syncerus long before, in the early Pliocene
or Miocene.
There are now a very large number of cattle breeds, varying in size, color,
sensitivity to heat, for milk or meat production, and so on. They all
have the same karyotype of 2n-60. Hybrids have been produced with gaur,
zebu, banteng, bison, gayal, and with yak (Gray, 1972). It is very dubious
that attempts at hybridization with the water buffalo (Bubalus)
have ever succeeded although it has often been tried.
 |
Cows at Munich Zoo. |
Twinning is not infrequent in cattle (0-5%) and the distribution over a variety of races has been summarized by Rutledge (1975). It results in smaller calves that are about 5 days premature and that have a much higher mortality (Cady & v. Vleck, 1978). Twin-bearing also increases with age and may thus be related to the known effects of maternal age and race, namely of elevated FSH/LH levels that have been defined in humans. It is thus also not surprising that twins and triplets can be obtained by the use of PMSG and hCG (Mauléon et al., 1970). Komisarek & Dorynek (2002) made an initial search for genes that may influence various twinning rates. They indicated that beef cattle have twins in 1% of their offspring while dairy breeds have 4-5%. It is an undesirable feature primarily because of the perinatal mortality but also because of the possibility of freemartinism (see section on endocrinology). Acardiac twins have also been reported in cattle twins (see section on pathology). Almost all twins are dizygotic (fraternal); monozygotic twinning appears to be rare in cows.
The cow has a 21 day estrus cycle with a 280 day length of gestation (270-285, depending on breed), much like humans. Singletons are commonest, twins occur in 1-5% and depending on breed; triplets are even rarer, and quadruplets are exceptional. The weight of cows and newborns varies very widely and depends largely on breed and nutritional state. Placentas weigh around 4-5 kg. A complete review of all aspects of cattle reproduction may be found in the chapter by Fogwell (1998).
3) Implantation
Wild (1964) described the sequence of implantation thus: After ovulation and fertilization the 2-cell stage is attained within 46-48 hours, the 4-cell stage in 56 hours, 8-cell stage in 72 hours and, after 96 hours, the morula enters the endometrial cavity, containing 16-32 cells. First cavitation occurs after 8-9 days with loss of zona pellucida. For the first 14 days the morula lengthens to become tubular. Implantation is preceded by the development of tiny papillary trophoblastic projections and occurs on day 19-20 post conception. A beautiful fine-structural study on the early interactions of trophoblast and endometrium was published by King et al. (1982). It shows the microvillous surfaces and early trophoblast penetration into the uterine epithelium. They found no evidence for transuterine migration in cows and indicated that, because of one's ability to flush and transfer blastocysts until day 16, that irreversible attachment does not occur prior to that day. Close attachment was found on day 19, beginning near the embryonic tissues but then spreading over the entire chorion, including the intercotyledonary regions. The maternal epithelium often becomes much thinned and may be absent in some regions, as degenerative products are also observed in the trophoblast. In text figure 2 these authors presented a schematic time table of the implantation processes, including the time of appearance of hormones (from days 10 to 32) that should be consulted for details.
4) General Characterization of the Placenta
The cow has a typical ungulate polycotyledonary placenta. The often-used term placentome defines the combination of cotyledon and maternal caruncle - the placental unit if you wish. It is very variable in size, shape, thickness, histologic composition and other features. Nevertheless, it is the principal unit and comparative analysis of that unit among bovines, let alone other species, is considered to be the important aspect of adjudicating relationships and adequacy for fetal growth. Mossman (1987) considered the bovine cotyledon to be "concave". The placenta usually intrudes into the other horn but has fewer cotyledons at that site.
There is a very large number of publications on the bovine placenta that have been reviewed by Mossman (1987). The controversial aspects to Mossman were the question of epithelio-chorial (alone) or (combined with) syndesmochorial placentation, and the origin of the binucleate cells. Similarly, Amoroso (1961), who cited all the evidence for or against the maintenance of the endometrial surface epithelium. He suggested, for instance, that most of the intercotyledonary endometrial epithelium disappears (except for the near-glandular regions (areolae). Also, that the epithelium in between the maternal fibrous (endometrial/caruncular) septa is replaced by trophoblast. But, there is also much controversy as he cites abundantly. Wooding & Flint (1994) described the relationship as a "synepitheliochorial" one because of their distaste for the term syndesmochorial and their belief that the binucleate cell becomes fused with endometrial epithelium (more about that below). They were shown to be migrating towards the maternal tissue. Their chapter has a very detailed consideration of this aspect with much fine-structural support.
The macroscopic aspects of cow placentas have been superbly studied by Wild (1964). That study summarized results of 308 cow placentas, reviewed the literature and concerned itself especially with the possibility that abnormal placentation can be a cause of neonatal infectious problems. Wild concludes that the average weight of cow placentas' chorions is 4,501 g; amnions weighed 590 g, the allantois 699 g. He then measured the surface of all cotyledons with a median of 4,474 cm2 - from 4,424 to 4794 cm2. There was a significant correlation of small surfaces with neonatal pneumonia and diarrhea. There is a remarkable variation in the number cotyledons, in cow placentas from 80 to 120. Indeed, Wild quotes authors who found only very few (3), large cotyledons in healthy offspring, others have stated that as many as 150 may occur. The number of cotyledons is generally determined by the number of maternal caruncles in the bicornuate uterus. These occur primarily in four rows through the uterine body, perhaps lower at the very peripheries. Similarly, the size of the cotyledons varies greatly, also being smaller at the periphery. Their greatest diameter was given as 16 cm. But also, on rare occasion, a bovine placenta is described as being "diffuse" rather than cotyledonary.
Testard & du Buisson (1970) also studied a large number of bovine placentomes of singletons and twin gestations. In singletons, there were generally fewer cotyledons in the non-occupied uterine horn than in the pregnant horn (34 to 73 +/-) but there was great variation in numbers. Laven & Peters (2001) made a more recent morphometric study of bovine placentas that generally corroborates former descriptions. Another recent study is that by Schlafer et al. (2001) that concentrates on the development of the placental macrophages.
Most studies on bovine placentas have been directed to the details of the fine structure of the cotyledons. Most authors agree that the bovine placenta is primarily an epithelio-chorial organ with possibly small regions of a syndesmochorial character. Baur (1972) compared the overall villous surface of the cow placenta with human placentas and found that it gradually rises to term (falling in late gestation slightly) but that it is roughly 10 times larger than that of the human placenta (commensurate with fetal weight - notwithstanding possible deductions from the Grosser classification). Hradecky et al. (1988a) measured the length of villi in different artiodactyl cotyledons and found those of cows to be 17 mm with extensive branching. The villi became more compact with advancing age and were covered with single-layered epithelium. Their figures also show clearly that the crypts are lined with flat to cuboidal epithelium.
Schmidt et al. (2006) have recently studied the placenta of Syncerus in detail and compared it with that of cattle. The difference in the construction of the cotyledons is very apparent from their study.
Villi are covered with a single layer of cuboidal trophoblast that are more cylindrical under the chorionic surface. The striking and oft-discussed feature is the large number (15-20%) of binucleate cells, definitely trophoblast in origin but with changing fates. Trophoblastic binucleate cells formed from trophoblast explants on type I collagen substrate (not film!) and produced placental lactogen (Nakano et al., 2002). While prior in vitro studies had shown binucleate cell production, this is the first study showing lactogen production in vitro. That these cells fuse with endometrial epithelium ("synepitheliochorial") has been demonstrated by Wooding & Flint (1994) in decisive electron microscopic studies. There is no doubt now that these cells "migrate" (move) toward the maternal tissues and release placental lactogen and probably some progesterone. They also degenerate and are (focally) replaced by endometrial epithelium. It seems to me useless to have a disagreement over whether this placental type should be called an epitheliochorial, synepitheliochorial, or syndesmochorial organ. Various relationships may apply at one time or another and may then have a functional impact or not. What seems to me more important is the finding that such multinucleate cells (they often fuse with endometrial cells and become trinucleate syncytia) appear to have the propensity of infiltrative movement (Wooding & Flint, 1994). They do so in a variety of quite different placentations, as was pointed out by the authors. I should point out though that this demonstration required excellent electron microscopy for detection. Wooding et al. (1997) emphasized that the possession of binucleate cells is one of the most characteristic shared features of artiodactyl placentation, 15-20% of the epithelium being binucleate cells. Here and elsewhere did they demonstrate the fusion with caruncular epithelium to make "hybrid" trinucleate cells and syncytial plaques, mostly near the apex of the crypts, as they called them. The fact that the granular content of the binucleate cell cytoplasm "streams" into the maternally-derived cytoplasm of the trinucleate cell, suggested to the authors that this brings the placental lactogen (of the binucleate cell) closer to the maternal circulation where it needs to be effective. Aspects on placental lactogen are more fully discussed in the chapter on Sheep placentation.
Recent evidence from cloning the gene of bovine placental heparanase and hybridization studies, Kizaki et al. (2003) have shown convincingly that this cell produces heparanase during early and late bovine gestation. These authors suggest that heparanase "may take migratory roles in placentogenesis for degrading the extracellular matrix".
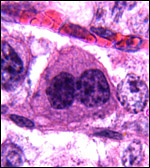 |
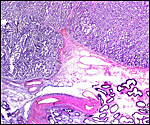
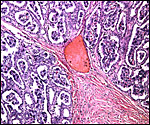
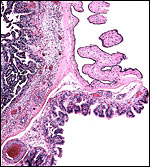
Typical binucleate trophoblast. |
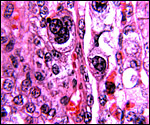 |
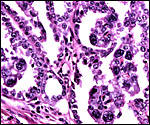
Trinucleate cell in maternal septum. |
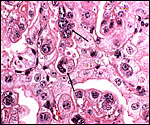 |
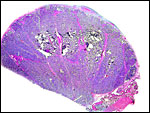
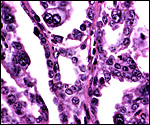
Immature cow placenta with multinucleate trophoblast at arrows. |
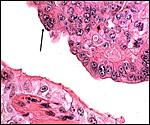 |
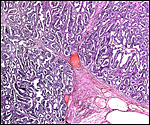
"Syncytial plaque" at apex of cow placental septum. |
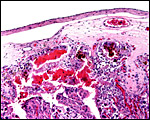 |
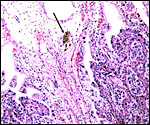
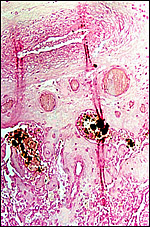
Subchorial hemorrhage adjacent to trophoblast with yellow-brown pigment. |
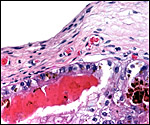 |
Higher magnification of previous photograph. |
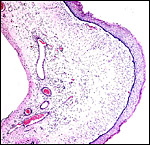
The umbilical cord has 4 large blood vessels, two arteries and two veins, in addition to a moderate number of smaller allantoic blood vessels. It also contains an allantoic duct connecting bladder via urachus with the allantoic sac. There are many squamous plaques on the surface of cattle umbilical cords.
 |
Term bovine umbilical cord with allantoic duct in the lower center. |
7)
Uteroplacental circulation
The chapter by Wooding and Flint (1994) includes a nice demonstration
of the villous capillary bed from latex injections. Ferrell & Reynolds
(1992) measured blood flows in fetal organs, cord and placenta in instrumented
cattle gestations. They found that the uterine blood flow of Hereford
cows was lower than that in Charolais, and lower when twin fetuses were
present than singletons. Other details need to be read in the original
contribution. More recently, Pfarrer et al. (2001) produced corrosion
casts and scanning electronmicroscopy to delineate the maternal and fetal
architecture of the bovine placenta. There are many details in that contribution
that must be studied from the paper to fully understand the complexity
of the vascular bed. Miglino & DiDio (1992) also did corrosion casts
but that publication was not accessible to me.
8) Extraplacental membranes
The amnion is avascular but often has areas of squamous metaplasia. The
allantoic sac is large and tubular; it is the recipient of fetal urination
and is vascularized. Steven (1975) depicted hippomanes obtained from bovine
placentas.
There is no infiltration of the maternal tissues unless one considers the fusion with focal migration of the binucleate cells in the epithelium of the clefts as invasion; I do not.
10)
Endometrium
There is no decidua. The ruminant endometrium is quite variable. It is
glandular in between the caruncular regions where areolae form but not
so in the caruncles. The caruncles are usually arranged in row; four in
cattle, perhaps fewer at their tubal ends, and fewer rows exist in deer
and some other artiodactyla. That is quite variable but determines the
ultimate shape of the placenta. The caruncles are composed of fibrous
thickening of the endometrial stroma with clefts into which the villi
ultimately extend.
Amoroso (1961) mentioned the descriptions of the presence of melanin in
endometrium of some ruminants but it is my impression that the yellow
trophoblastic pigment he described is still believed to be a residual
of focal hemorrhages at the tips of the endometrial septa.
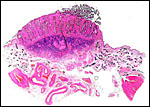 |
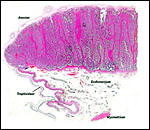
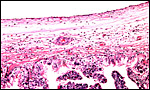
Bovine endometrial caruncle. The dark blue fibrous tissue and the septa (pink) with epithelial lining are well delineated. |
There is no subplacenta and no other special features exist.
12)
Endocrinology
Because of the importance of cattle reproduction, there are numerous studies
on endocrine profiles during pregnancy, abortion, dystocia, etc. The onset
of parturition has been investigated with changes in prostaglandin levels,
progesterone and placental lactogen levels, and the normal hormone concentrations
have been charted by Challis et al., 1974). Fetal concentrations of progesterone
were much lower than in the dam; testosterone (and LH) was significantly
higher in male than female fetuses, but not those of androstenedione or
progesterone. Kim et al. (1972) had earlier demonstrated high levels of
testosterone in male fetal calves and believed it to be one possible mechanism
for the freemartin effect (see also Short, 1970). The high levels of estrogen
in the fetal circulation were interpreted as coming from the maternal
ovary and placenta. Some relevant information is also referenced by Kindahl
et al. (2002) who were interested in monitoring fetal well-being. Because
the function of placental steroids was unresolved, Hoffmann & Schuler
(2002) ascertained the location of their placental and caruncular receptors.
They deduced that estrogens and progesterone from the placenta (in contrast
to the corpus luteum which is necessary for maintenance of gestation)
are "important factors controlling caruncular growth, differentiation
and function."
Kassam et al. (1987) measured progesterone and prostaglandin (PGF2a)
in cows whose fetuses were killed on day 46 by manual rupture of the amnionic
vesicle. Basically, in this sterile fetal demise, pregnancies continued
and progesterone was maintained until luteolysis occurred when the prostaglandin
levels increased. PGF2a did not rise solely because of fetal demise. Nakano
et al. (2002) cultured bovine trophoblast on Type I collagen gels and
saw it differentiate, i.a. into binucleate cells with placental
lactogen expression.
13)
Genetics
Cattle have 60 chromosomes, all autosomes are acrocentrics, the sex chromosomes
are metacentrics. An extensive literature exists on these animals' chromosomes,
some of which was summarized by Hsu & Benirschke (1967) and in the
subsequent editions of these volumes. The Y-chromosome is entirely heterochromatic.
An internationally accepted nomenclature of the chromosomes was published
(ISCNDB, 2000) and is frequently referred to when artiodactyl chromosome
morphology and rearrangements are considered. A common chromosomal translocation
in cattle (##1/29) was first described by Gustavsson (1966), and this
has since been widely investigated (Amrud, 1969; Popescu & Pech, 1991).
It is associated with a minor reduction of fertility and the production
of some anomalies (Logue & Harvey, 1978; Gustavsson, 1980). Several
chromosomal pericentric inversions are summarized by Switonski (1987);
two cases of trisomy X were published (Buoen & Seguin, 1981); the
equivalent of Klinefelter's syndrome (61,XXY) with sterility was found
by Dunn et al. (1980); a sterile bull with XY/XYY mosaicism was described
by Miyake et al. (1984), and a few other Robertsonian fusions have come
to light that are summarized by Iannuzzi et al. (2001) who described a
sterile, normal appearing bull with a #9/Y fusion chromosome. Robertsonian
fusion of chromosomes 3 and 4 was described by Popescu (1977a,b) who also
reviewed the literature up that year. Hare et al. (1980) studied 159 embryos
during embryo transfers and found several polyploid and mosaic polyploid
embryos. Not surprisingly, there are numerous errors (hypo-, hyper-diploidy)
in bovine leukemias and lymphosarcoma (Hare et al., 1964)
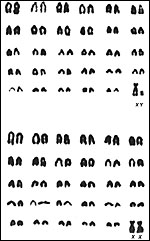 |
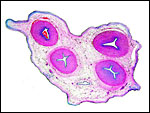
Karyotypes of male and female cattle. |
Hill et al. (2002) showed that the trophoblast of cloned cow embryonic placentas express frequently a high degree of MHC-I expression (in contrast to normal implantations) and that this is accompanied by a strong lymphocytic (T-cell) infiltration of the endometrium. Schlafer et al. (2000) studied the development of placental macrophages in cow placentas and showed a steady and remarkable increase of them with advancing gestation. They are the equivalent of human "Hofbauer cells."
15) Pathological features
Holm et al. (1965) studied eight cotyledons of different cows with prolonged pregnancy. They showed fibrosis of maternal septa, atrophy of trophoblast, fibrosis of villi and, at times, sclerosis of maternal arteries. I have seen several acardiac fetuses in cattle twins and show one such anomaly here. They result from a reversal of the circulation in one of twins via placental anastomoses and are well-studied by Schatz and others (references can be found in Benirschke & Harper, 1977). They take many forms but are not to be confused with teratomas (see Stephens et al., 1989). Most acardiacs have a short umbilical cord, but it may be so short that it is missed. They cannot be confused with teratomas because their outside is composed of skin, a feature not found in teratomas. Dunn et al. (1967) studied the chromosomes of one such female twin that was blood chimeric to a normal male co-twin.
 |
Typical acardiac twin fetus in cattle. "Face" is left. There is even a tail. |
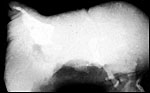 |
Radiograph of the same fetus. "Head" is left, deformed legs on right. |
Meinecke
et al. (2002) presented a case of "hydatidiform mole" in a bovine
freemartin, but the case is quite confusing. It shows a stillborn male fetus
(60,XY) with large attached 47.8 kg "twin mole" with a 60,XX karyotype.
There were no fetal remnants and the "mole" was composed of fluid-filled
cisterns. To equate this to an androgenetic origin (as are human moles)
seems to be overinterpreting the findings. Indeed, whether the few cases
cited by the authors really are similar in their pathogenesis to human moles
is dubious.
Uterine infections are common in cattle and often the cause of infertility.
A special organism here is Tritrichomonas sp., but other organisms are frequently
identified (Edwards & Wikse, 2002). Male sterility is frequently due
to testicular hypoplasia, cryptorchidism and sperm anomalies, extensively
studied in a Danish population by Christensen (1965). Anencephaly was described
in four calves by Cho & Leipold (1978). Epizootic outbreaks of congenital
arthrogryposis and hydranencephaly (possibly due to Akabane virus) has been
studied in Australia by Shepherd et al. (1978).
It is not surprising that, because of the large number of cattle in the
world and because of the economic importance of this animal, numerous diseases
and various other pathological conditions have been described. They are
far too numerous to be cited here. Infections play a major role as cause of abortions, aptly summarized by Schlafer (2002). He also reviewed the frequent placental retention after delivery. Brucellosis, many other infectious diseases
such as malignant catarrhal fever can affect cattle seriously. The latter
may be transferred from sheep, and wildebeest (Heuschele et al., 1984).
There is also a host of neoplastic diseases such as leukemia, lymphoma,
and many congenital abnormalities other than those already listed. A convenient
book in which to gain access to some of this information is that written
by McEntee (1990).
Gerneke & de Boom (1967) described the "snaring" of the allantois
with embryonic death and malformations by what they considered to be remnants
of regressing yolk sac. Corcoran & Murphy (1965) described a very large
placental tumor ("3 stone") that was most likely a chorangioma.
Edwards (2002) described three ovarian epidermoid cysts in cows.
16)
Physiologic data
Lischer & Steiner (1993, 1994) did sonography of the involution of
umbilical structures (vein, urachus) following delivery because of the
occurrence of umbilical hernias and other problems and to direct proper
therapy. To advance neonatal surgery on umbilical problems, Boure et al.
(2001) showed that endoscopic resection of the bladder apex can safely
be performed. Comline & Silver (1976) chronically instrumented cows
during gestation and determined biological gradients between mother and
fetus, as well as a variety of flows and gases. Ferrell & Reynolds
(1992) likewise, instrumented fetuses and dams (see section on blood flow
above).
17)
Other resources
Numerous strains of cattle fibroblasts exist in many laboratories or can
be obtained from the ACC.
18)
Other remarks - What additional information is needed?
Cloning effects: After the sheep "Dolly" was produced
from cloned somatic cells of the breast of a sheep, several laboratories
have succeeded in cloning cattle fetuses (and other species) from somatic
cells (Campbell et al., 1996). Kato et al. (1998) reported eight calves
born from somatic cells with an 80% success rate. Kubota et al. (2000)
reported on six cloned calves; Lanza et al. (2001) had a 45% implantation
rate but found several problems also. These are discussed in this large
experiment; still, they had 24 Holstein cows survive. But a variety of
novel problems have also arisen. In embryo transfers between gaur and
cows, Hammer et al. (2001) already found fewer placental cotyledons than
expected, and they were much larger in size; moreover, the neonates had
major stability problems. Likewise, Bertolini et al. (2002) found that
placentation results from in vivo- vs. in vitro-produced bovine
pregnancies yielded thinner and longer placentomes in the in vitro group. Other abnormal gestational features were also identified. Hill
et al. (1999) reported placental edema, neonatal respiratory distress,
cardiomyopathy and pulmonary hypertension in some of their transgenically
produced calves. Later, they showed that placental problems (rudimentary
development) appeared to be the major cause of the prenatal demise of
cloned calves (Hill et al., 2000, 2001). Hashizume et al. (2002) found
fewer placentomes at day 60 in cloned animals and abnormal placentomes.
There was a "disparate expression of genes and proteins" when
compared to normal implantations. Placental lactogen and pregnancy-associated
glycoproteins were altered. They suggested that, as pregnancy advances,
this altered gene expression was eliminated. Heyman et al. (2002) observed
that maternal pregnancy serum specific protein (PSP60) was significantly
higher in failing cloned gestations after day 50 than in controls, with
developing hydroallantois and larger placentomes being a major problem.
Chavatte-Palmer et al. (2002) also found placental edema and lower placentome
numbers (69.9 vs. 99) and greater weights (144.3 g vs. 137 g) in cloned
offspring. It has thus been speculated that imprinting errors may be one
reason for placental abnormalities. Koo et al. (2002), on the other hand
suggested that both for cloned and in vitro-produced cows there was an
inappropriate number cells allocated between trophoblast and embryo, with
the embryo being significantly short-changed in both situations when compared
with normal in vivo conceptuses. This, they felt, was the possible reason
for frequent fetal losses in early development. Hill et al. (2002) found
that cloned embryos' trophoblast expressed MHC-I antigens in between 17.9
and 56.5%, while no normal conceptuses did. Moreover, there were an increased
number of T-cells in the endometrium. Sato et al. (2002) demonstrated
increased fragility of the umbilical cords and fine-structural alterations
of the cotyledons in cloned conceptuses.
Interspecific cloning has also been attempted, preparing for knowledge
to be obtained for animal transfers from endangered species in zoos. Thus,
following a live birth obtained by embryo transfer from gaur into cow
(Pope et al., 1988; but also see Hammer et al., 2001), Lanza et al. (2000)
reported a live gaur birth by cloning from somatic cells using cow recipients.
Embryo transfer from gaur into cow has not always been successful, however,
and some structural placental abnormalities have been identified (Hradecky
et al., 1988b). There was inadequate branching of villi and inadequate
endometrial epithelial development. We have a long way to go before the
fine details are understood.
Finally, some comparative data from related species, especially those
with which cattle can hybridize are needed. Thus, the Banteng chapter
of this series may be of interest. That species had fewer cotyledons.
I have had one unusually large cotyledon given to me by a hunter of a
pregnant bison he had shot. Regrettably he did not enumerate the number
of placentomes, but the single cotyledon was at least three times larger
than the largest cow structure I had seen. Structurally it was very similar,
contained some yellow pigment (iron-negative) in subchorial trophoblast
as other ungulates, and the umbilical cord had its usual four large and
many small blood vessels, surface callosities and the allantoic duct.
 |
Isolated cotyledon from American bison. |
References
Amoroso, E.C.: Placentation. Chapter 15, pp.127-311, In, Marshall's Physiology of Reproduction, V.II, A.S. Parkes, ed. Second Edition. Little, Brown & Co. Boston, 1961.
Amrud, J.: Centric fusion of chromosomes in Norwegian red cattle (NRF). Hereditas 62:293-302, 1969.
Baur, R.: Quantitative Analyse des Wachstums der Zottenoberfläche bei der Placenta des Rindes und des Menschen. Z. Anat. Entwicklgs.-Gesch. 136:87-97, 1972.
Benirschke,
K.: Spontaneous chimerism in mammals. A critical review. Current Topics
in Pathol. 51:1-61, 1970 (Springer-Verlag, NY).
Benirschke,
K. and Bourne, G.L.: The incidence and prognostic implication of congenital
absence of one umbilical artery. Amer. J. Obstet. Gynecol. 79:251?254,
1960.
Benirschke, K. and Harper, V.D.R.: The acardiac anomaly. Teratology 15:311?316, 1977.
Bertolini, M., Mason, J.B., Beam, S.W., Carneiro, G.F., Sween, M.L., Kominek, D.J., Moyer, A.L., Famula, T.R., Sainz, R.D. and Anderson, G.B.: Morphology and morphometry of in vivo- and in vitro-produced bovine concepti from early pregnancy to term and association with high birth weights. Theriogenology 58:973-994, 2002.
Björkman, N.: Morphological and histochemical studies on the bovine placenta. Acta Anat. 22: Supppl.2, 1-91, 1954.
Björkman, N. and Sollen, P.: Morphology of the bovine placenta at normal delivery. Acta Vet. Scand. 1:347-362, 1960.
Boure, L., Foster, R.A., Palmer, M. and Hathway, A.: Use of an endoscopic suturing device for laparoscopic resection of the apex of the bladder and umbilical structures in normal neonatal calves. Vet. Surg. 30:319-326, 2001.
Buoen, L.C. and Seguin, B.E.:X-trisomy karyotype and associated infertility in a Holstein heifer. JAVMA 179:808-811, 1981.
Cady, R.A. and van Vleck, L.D.: Factors affecting twinning and effects of twinning in Holstein dairy cattle. J. Anim. Sci. 46:950-956, 1978.
Campbell, K.H.S., McWhir, J., Ritchie, W.A. and Wilmut, I.: Sheep cloned by nuclear transfer from a cultured cell line. Nature 380:64-66, 1996.
Challis, J.R., Kim, C.K., Naftolin, F., Judd, H.L., Yen, S.S. and Benirschke, K.: The concentrations of androgens, oestrogens, progesterone and luteinizing hormone in the serum of foetal calves throughout the course of gestations. J. Endocrinol. 60:107-115, 1974.
Chavatte-Palmer, P., Heyman, Y., Richard, C., Monget, P., LeBourhis, D., Kann, G., Chilliard, Y., Vignon, X. and Renard, J.P.: Clinical, hormonal, and hematologic characteristics of bovine calves derived from nuclei from somatic cells. Biol. Reprod. 66:1596-1603, 2002.
Cho, D.Y. and Leipold, H.W.: Anencephaly in calves. Cornell Vet. 68:60-69, 1978.
Christensen, N.O.: Erbliche Sterilität beim Rinde. Fortpfl. Haust. 2:145-166, 1965.
Comline,
R.S. and Silver, M.: Some aspects of foetal and Uteroplacental metabolism
in cows with indwelling umbilical and uterine catheters. J. Physiol. 260:571-586,
1976.
Corcoran, C.J. and Murphy, E.C.: Rare bovine placental tumour - A case
report. Vet. Rec. 77:1234-1235, 1965.
Dunn, H.O., Lein, D.H. and Kenney, R.M.: The cytological sex of a bovine anidian (amorphous) twin monster. Cytogenetics 6:412-419, 1967.
Dunn, H.O., Lein, D.H. and McEntee, K.: Testicular Hypoplasia in a Hereford bull with 61,XXY karyotype: The bovine counterpart of human Klinefelter's syndrome. Cornell Vet. 70:137-146, 1980.
Edwards,
J.F. and Wikse, S.E.: Lesions of spontaneous bovine uterine trichomoniasis.
Vet. Pathol. 39 (5) 621, abstract # 35, 2002.
Edwards, J.E.: Three cases of ovarian epidermoid cysts in cattle. Vet.
Pathol. 39:744-746, 2002.
Ferrell, C.L. and Reynolds, L.P.: Uterine and umbilical blood flows and net nutrient uptake by fetuses and Uteroplacental tissues of cows gravid with either single or twin fetuses. J. Anim. Sci. 70:426-433, 1992.
Fogwell,
R.: Cattle. In, Encyclopedia of Reproduction, E. Knobil and J.D. Neill,
eds., Academic Press, San Diego, Vol.1:510-525, 1998.
Gerneke, W.H. and de Boom, H.P.A.: Omphalo-allantoic snaring causing bovine
embryonal pathology: A case report. J. South Afr. Vet. Med. Assoc. 38:241-244,
1967.
Gray,
A.P.: Mammalian Hybrids. A Check-list with Bibliography. 2nd
edition.
Commonwealth Agricultural Bureaux Farnham Royal, Slough, England, 1972.
Groves, C.P.: Systematic relationships in the Bovini (Artiodactyla, Bovidae). Z. zool. Systematik Evol.-fschg. 19:264-278, 1981.
Gustavsson, I.: Chromosome abnormality in cattle. Nature 211:865-866, 1966.
Gustavsson, I.: Chromosome aberrations and their influence on the reproductive performance of domestic animals. A review. Z. Tierz. Zuchtbiol. 97:176-195, 1980.
Hammer, C.J., Tyler, H.D., Loskutoff, N.M., Armstrong, D.L., Funk, D.J., Lindsey, B.R. and Simmons, L.G.: Compromised development of calves (Bos gaurus) derived from in vitro-generated embryos and transferred into domestic cattle (Bos taurus). Theriogenology 55:1447-1455, 2001.
Hare, W.C.D., McFeely, R.A., Abt, D.A. and Feierman, J.R.: Chromosomal studies in bovine lymphosarcoma. J. Nat. Cancer Inst. 33:105-118, 1964.
Hare, W.C.D., Singh, E.L., Betteridge, K.J., Eaglesome, M.D., Randall, G.C.B., Mitchell, D., Bilton, R.J. and Trounson, A.O.: Chromosomal analysis of 159 bovine embryos collected 12 to 18 days after estrus. Can. J. Genet. Cytol. 22:615-626, 1980.
Hashizume, K., Ishiwata, H., Kizaki, K., Yamada, O., Takahashi, T., Imai, K., Patel, O.V., Akagi, S., Shimizu, M., Takahashi, S. Katsuma, S., Shiojima, S., Hirasawa, A., Tsujimoto, G., Todoroki, J. and Izaike, Y.: Implantation and placental development in somatic cell clone recipient cows. Cloning Stem Cells 4:197-209, 2002.
Heuschele, W.P., Fletcher, H.R., Oosterhuis, J., Janssen, D. and Robinson, P.T.: Epidemiologic aspects of malignant catarrhal fever in the USA. Proc. 88th Ann. Meet. US Anim. Health Assoc. pp.640-651, 1984.
Heyman, Y., Chavatte-Palmer, P., LeBourhis, D., Camous, S., Vignon, X. and Renard, J.P.: Frequency and occurrence of late-gestation losses from cattle cloned embryos. Biol. Reprod. 66:6-13, 2002.
Hill, J.R., Roussel, A.J., Cibelli, J.B., Edwards, J.F., Hooper, N.L., Miller, M.W., Thompson, J.A., Looney, C.R., Westhusin, M.E., Robl, J.M. and Stice, S.L.: Clinical and pathologic features of cloned transgenic calves and fetuses (13 case studies). Theriogenology 51:1451-1465, 1999.
Hill, J.R., Burghardt, R.C., Jones, K., Long, C.R., Looney, C.R., Shin, T., Spencer, T.E., Thompson, J.A., Winger, Q.A. and Westhusin, M.E.: Evidence for placental abnormality as the major cause of mortality in first-trimester somatic cell cloned bovine fetuses. Biol. Reprod. 63:1787-1794, 2000.
Hill, J.R., Edwards, J.F., Sawyer, N., Blackwell, C. and Cibelli, J.B.: Placental anomalies in a viable cloned calf. Cloning 3:83-88, 2001.
Hill, J.R., Schlafer, D.H., Fisher, P.J. and Davies, C.J.: Abnormal expression of trophoblast major histocompatibility complex class I antigens in cloned bovine pregnancies is associated with a pronounced endometrial lymphocytic response. Biol. Reprod. 67:55-63, 2002.
Hoffmann, B. and Schuler, G.: The bovine placenta; a source and target of steroid hormones: observations during the second half of gestation. Domest. Anim. Endocrinol. 23:309-320, 2002.
Holm, L.W., Salvatore, C. and Zeek-Minning, P.: The histology of the postterm bovine placenta. Amer. J. Obstet. Gynecol. 88:479-489, 1964.
Hradecky, P., Mossman, H.W. and Stott, G.G.: Comparative histology of antelope placentomes. Theriogenology 29:693-729, 1988a.
Hradecky, P., Stover, J. and Stott, G.G.: Histology of a heifer placentome after interspecies transfer of a gaur embryo. Theriogenology 30:593-604, 1988b.
Hsu, T.C. and Benirschke, K.: Bos taurus. Vol. 1, Folia 44, in An Atlas of Mammalian Chromosomes. Springer-Verlag, N.Y. 1967.
Iannuzzi, L., Molteni, L., Di Meo, G.P., De Giovanni, A., Perucatti, A., Succi, G., Incarnato, D., Eggen, A. and Cribiu, E.P.: A case of azoospermia in a bull carrying a y-autosome reciprocal translocation. Cytogent. Cell Genet. 95:225-227, 2001.
Kassam, A., BonDurant, R.H., Basu, S., Kindahl, H. and Stabenfeldt, G.H.: Clinical and endocrine responses to embryonic and fetal death induced by manual rupture of the amniotic vesicle during early pregnancy in cows. JAVMA 191:417-420, 1987.
Kato, Y., Tani, T., Sotomuru, Y., Kurokawa, K., Kato, J-y., Doguchi, H., Yasue, H. and Tsunoda, Y.: Eight calves cloned from somatic cells of a single adult. Science 282:2095-2098, 1998.
Keller, K. and Tandler, J.: Über das Verhalten der Eihäute bei der Zwillingsträchtigkeit des Rindes. Wien. tierärztl. Mschr. 3:513-527, 1916.
Kim, C.K., Yen, S.C.C. and Benirschke, K.: Serum testosterone in fetal cattle. Gen. Comp. Endocr. 18:404-407, 1972.
Kindahl, H., Kornmatitsuk, B., Konigsson, K. and Gustafsson, H.: Endocrine changes in late bovine pregnancy with special emphasis on fetal well-being. Domest. Anim. Endocrinol. 23:321-328, 2002.
King,
G.J., Atkinson, B.A. and Robertson, H.A.: Implantation and early placentation
in domestic ungulates. J. Reprod. Fert. Suppl. 31:17-30, 1982.
Kizaki, K., Yamada, O., Nakano, H., Takahashi, T., Yamauchi, N., Imai,
K. and Hashizume, K.: Cloning and localization of heparanase in bovine
placenta. Placenta 24:424-430, 2003.
Klisch, K., Hecht, W., Pfarrer, C., Schuler, G., Hoffmann, B. and Leiser, R.: DNA content and ploidy level of bovine placentomal trophoblast giant cells. Placenta 20:451-458, 1999.
Komisarek, J. and Dorynek, Z.: Genetic aspects of twinning in cattle. J. Appl. Genet. 43:55-68, 2002.
Koo, D.B., Kang, Y.K., Choi, Y.H., Park, J.S., Kim, H.N., Oh, K.B., Son, D.S., Park, H., Lee, K.K. and Han, Y.K.: Aberrant allocation of inner cell mass and trophectoderm cells in bovine nuclear transfer blastocysts. Biol. Reprod. 67:487-492, 2002.
Kubota, C., Yamakuchi, H., Todoroki, J., Mizoshita, K., Tabara, N., Barber, M. and Yang, X.: Six cloned calves produced from adult fibroblast cells after long-term culture. PNAS 97:990-995, 2000.
Lanza, R.P., Cibelli, J.B., Diaz, F., Morales, C.T., Farin, P.W., Farin, C.E., Hammer, C.J., West, M.D. and Damiani, P.: Cloning of an endangered species (Bos gaurus) using interspecies nuclear transfer. Cloning 2:79-90, 2000.
Lanza, R.P., Cibelli, J.B., Faber, D., Sweeney, R.W., Henderson, B., Nevala, W., West, M.D. and Wettstein, P.J.: Cloned cattle can be healthy and normal. Science 294:1893-1894, 2001.
Laven, R.A. and Peters, A.R.: Gross morphometry of the bovine placentome during gestation. Reprod. Domest. Anim. 36:289-296, 2001.
Lillie, F.R.: The freemartin: a study of the action of sex hormones in the foetal life of cattle. J. Exp. Zool. 23:371-452, 1917.
Lischer, C.J. and Steiner, A.: Ultrasonography of umbilical structures in calves. Part I.: Ultrasonographic description of umbilical involution in clinically healthy calves. Schweiz. Arch. Tierheilk. 135:221-230, 1993.
Lischer, C.J. and Steiner, A.: Ultrasonography of the umbilicus in calves. Part 2: Ultrasonography, diagnosis and treatment of umbilical diseases. Schweiz. Arch Tierheilk. 136:227-241, 1994.
Mauléon, P., Bosc, M.-J., Courot, M., Pelot, J., Schneberger, J. and Ortavant, R.: Obtention de naissances gémellaires après superovulation limitée et charactéristiques zootechniques des vaches productrices et des vaches jumeaux. Annales de Biologie Animale, Biochemie, Biophysique 10:113-122, 1970 (Inst. Nat. Recherche Agron., Paris).
McEntee, K.: Reproductive Pathology of Domestic Mammals. Academic Press, San Diego, 1990.
Meinecke, B., Kuiper, H., Drögemüller, C., Leeb, T. and Meinecke-Tillmann, S.: A mola hydatidosa coexistent with a foetus in a bovine freemartin pregnancy. Placenta 24:107-112, 2002.
Miglino, M.A. and DiDio, L.J.: Vasculature of bovine placentas studied by scanning electron microscopy of corrosion casts. Ital. J. Anat. Embryol. 97:23-35, 1992.
Miyake, Y.-I., Kanagawa, H. and Ishikawa, T.: Further chromosomal and clinical studies on the XY/XYY mosaic bull. Jpn. J. Vet. Res. 32:9-21, 1984.
Mossman, H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Nakano, H., Shimada, A., Imai, K., Takezawa, T., Takahashi, T. and Hashizume, K.: Bovine trophoblastic cell differentiating on collagen substrate: formation of binucleate cells expressing placental lactogen. Cell Tissue Res. 307:225-235, 2002.
Pfarrer, C., Ebert, B., Miglino, M.A., Klisch, K. and Leiser, R.: The three-dimensional feto-maternal vascular interrelationship during early bovine placental development: a scanning electron microscopical study. J. Anat. 198:591-602, 2001.
Pilgrim, G.E.: The evolution of the buffaloes, oxen, sheep and goats. J. Linn. Soc. (Zool.) 41:272-286, 1947.
Pope, C.E., Dresser, B.L., Kuehn, G., Kramer, L. and Gillespie, D.: Live birth of a gaur (Bos gaurus) calf following nonsurgical embryo transfer to a Holstein (Bos Taurus) recipient. Theriogenology 29:289, 1988.
Popescu, C.P.: Les anomalies chromosomiques des bovins (Bos taurus L.). État actuel des connaissances. Ann. Génét. Sél. Anim. 9:463-470, 1977a.
Popescu, C.P.: A new type of Robertsonian translocation in cattle. J. Hered. 68:139-142, 1977b.
Rutledge, J.J.: Twinning in cattle. J. Anim. Sci. 40:803-815, 1975.
Sato, M., Kubota, C., Tanaka, S. and Onitsuka, T.: Pathological characteristics of the calves cloned from somatic cells. Vet. Pathol. 39:629 (abstract 66), 2002.
Schlafer, D.H., Fisher, P.J. and Davies, C.J.: The bovine placenta before and after birth: placental development and function in health and disease. Anim. Reprod. Sci. 60-61:145-160, 2000.
Schlafer, D.H.: Placental development, anatomy and pathology: Before and after birth. BCVA (Cattle Practice) 10 (Part 2): 169-174, 2002.
Schmidt, S., Gerber, D., Soley, J.T., Aire, T.A. and Boos, A.: Histo-morphology of the uterus and early placenta of the African buffalo (Syncerus caffer) and comparative placentome morphology of the African buffalo and cattle (Bos taurus). Placenta 27:899-911, 2006
Shepherd, N.C., Gee, C.D., Jessep, T., Timmins, G., Carroll, S.N. and Bonner, R.B.: Congenital bovine epizootic arthrogryposis and hydranencephaly. Austral. Vet. J. 54:171-177, 1978.
Short, R.V.: The bovine freemartin: a new look at an old problem. Phil Trans. Roy. Soc. B.259:141-147, 1970.
Stein, F.J. and Martin, J.E.: Single umbilical artery and other congenital defects in a calf. Anat. Anz. 159:369-372, 1985.
Steven, D.H.: Comparative Placentation. Essays in Structure and Function. Academic Press, London, 1975.
Switonski, M.: A pericentric inversion in an X chromosome in the cow. J. Hered. 78:58-59, 1987.
Testart, J., du Mesnil du Buisson F.: Étude biométrique des placentomes dans les gestations simples ou gémellaires des bovins. Annales de Biologie Animale, Biochemie, Biophysique 10:87-97, 1970 (Inst. Nat. Recherche Agron., Paris).
Visscher, P.M., Smith, D., Hall, S.J.G. and Williams, J.A.: A viable herd of genetically uniform cattle. Nature 409:303-304, 2001.
Wild, A.: Untersuchungen uber den Aufbau der Placenta fetalis des Rindes und ihre Auswirkung auf die Gesundheit des Kalbes. Zbl. Veterinärmediz. B, 11:60-89, 1964.
Wooding, F.B.P. and Flint, A.P.F.: Placentation. Chapter 4 (pp.233-460) in, G.E. Lamming, ed. Marshall's Physiology of Reproduction, 4th ed. Vol. 3, Part 1. Chapman & Hall, London, 1994.
Wooding, F.B., Morgan, G. and Adam, C.L.: Structure and function in the ruminant synepitheliochorial placenta: central role of the trophoblast binucleate cell in deer. Microsc. Res. Tech 38:88-99, 1997.