| (Clicking
on the thumbnail images below will launch a new window and a larger
version of the thumbnail.) |
| Last updated: Feb. 28, 2004. |
Syncerus caffer nanus (nana)
Order: Artiodactyla
Family: Bovidae
1) General Zoological Data
There is one species of African buffalo, Syncerus caffer. The Forest buffalo that is being considered here, Syncerus caffer nanus, while physically quite different, is generally listed as a subspecies although it is very distinct phenotypically and in its habitat. The Savannah buffalo, Syncerus caffer caffer is the larger, darker species and considered to be an extremely dangerous game animal in Africa. Pohle (2002) described that the aggressiveness in captivity has even led to a number of conspecific deaths. Few zoos keep this species now; there are only three left in Germany that keep this species (Pohle, 2002). Numerous different subspecies have been considered to characterize the intermediate forms; these were reviewed extensively by Grubb (1971) and are also listed by Wilson & Reeder (1992). It appears to me that there is still considerable confusion in this genus with its numerous phenotypes, the formerly wider distribution and, especially, because of the wide variability of karyotypes (52-56) which is atypical for any "good" species. Perhaps the results of mtDNA by Simonsen et al. (1998) give further explanation to these karyotypic varieties. These authors found a "high levels of genetic variability" that did not support suggestions that severe bottlenecks had occurred in recent decades as a possible result of outbreaks of rinderpest. "Hybridization" occurs occasionally between Savannah and Forest buffalos but this alone can hardly account for the wide phenotypic differences.
The Forest buffalo is smaller ("dwarf" = nanus) and has longer red-brown hair; the Savannah species is larger and darker as well as less hairy. In between, but also of Western origin, are red, less hairy buffalos with different names (e.g. S. c. brachyceros). Groves (1981) reviewed what is known of bovid speciation and placed the divergence of Syncerus- from Bos-like ancestors at about 4-5 million years ago. Many zoological gardens exhibit one or the other species. The animals may live up to 30 years.
 |
Congo (forest) buffalo at San Diego Zoo. |
 |
Congo (forest) buffalo at San Diego Zoo. |
2)
General Gestational Data
Grimsdell (1973) has described the reproductive history of Syncerus.
Gestational length is given as between 300 and 345 days, but Sinclair
(1977) in his lengthy study, describes it as being on average 340 days.
Knechtel (1993) gives 11.2 months as the average length of gestation.
Reproduction commences at age 3 years, both for cows and bulls (Pohle,
2002). Singletons are the rule and, in S. c. caffer, newborns weigh
around 40 kg. First births are at 4 years and births are spaced by two
years.
3)
Implantation
Mossman (1987) was emphatic that in all the bovids that he had examined,
including the African buffalo, implantation of the umbilical cord occurs
mesometrially, in a midcornual location. This indicated to him that this
is the place at which the blastocyst comes to rest for elongation and
implantation. He also described in considerable detail the development
of cotyledons and of the uterine caruncles. In general, this specimen
of Syncerus conforms to that description.
4)
General Characterization of the Placenta
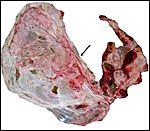
This is a multicotyledonary, epithelio-chorial placenta with extension
over both uterine horns. I have had two specimens available which were,
unfortunately somewhat torn and autolyzed. One weighed 2,200 g, possessed
52 very flat cotyledons that measured between 4 and 10 cm in dimension
and 0.5 cm in thickness. The cotyledons were larger and more widely spread
in the horn occupied by the fetus than in the empty horn. The cord inserted
centrally, had 4 vessels and an allantoic duct. It was 9 cm long. The
other placenta comes from a 29.9 kg neonate and weighed 2,575 g, measured
217 x 48 cm and had 54 flat cotyledons. They varied from 8 to 6 cm. This
umbilical cord measured 28.7 x 0.8 cm. Both specimens were accompanied
by hippomanes. Thus, both specimens I have been able to study had fewer
cotyledons than are found in cows (+/80) and they were also flatter than
seen in cattle (Wild, 1964).
Since this description a neonatal demise following dystocia was recorded.
That placenta weighed 2,250 g and had 34 very flat cotyledons measuring
up to 6.5 cm. The cord was 35 cm long. The cause of death was probably
related to the placental infection shown below.
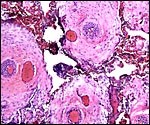
As other Bovoidea, Syncerus has a single-layered, single-nucleated trophoblast cover over its villi, with the exception of the typical binucleate cells that are so characteristic for ruminants (Wooding, 1982). These were studied in greater detail by Wooding et al. (1997) and are now considered to produce placental lactogen and other glycoproteins. Their glycoprotein molecule production was studied by Atkinson et al. (1993) but their usefulness is as yet unknown.
One of the umbilical cords measured 9 cm, the other 28.7 cm. They had four large blood vessels and a central allantoic duct. The cords had essentially no Wharton's jelly, no twists and no other unusual features. The last specimen received following Cesarean section had a 35 cm long umbilical cord.
7) Uteroplacental circulation
This has not been described for this species.
8)
Extraplacental membranes
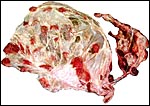
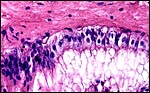
Both placentas had a 5 cm large yellow hippomanes. They were found in
the long, narrow allantoic sac that is typical for this species. They
consisted of central amorphous material with a coating of numerous colorless
crystals. The trophoblastic epithelium as well as the amnionic epithelium
of both placentas was autolyzed.
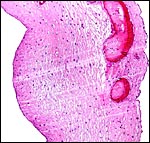 |
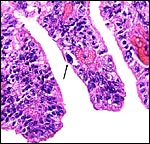
Allanto-amnion, with vascularized allantoic membrane at right. Amnion at left. |
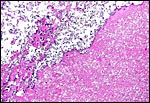 |
Hippomanes with crystalline surface deposit at left. |
Since no implanted placenta has been available, no judgment can be made as to uterine trophoblast infiltration. Considering other bovid specimens, however, invasion of the endometrium is unlikely in this species as well.
10)
Endometrium
Unknown.
11)
Various features
Unknown.
12)
Endocrinology
Females have a 23 day estrous cycle and estrus lasts 5-6 days.
13)
Genetics
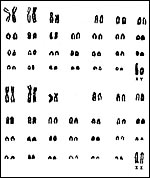
The Congo buffalo, Syncerus caffer nanus, was thought to have 54
chromosomes when we examined specimens from the Catskill Game Farm, while
its relative, Syncerus caffer caffer had 52 chromosomes (Wurster
& Benirschke, 1968). The difference being a Robertsonian fusion element,
# 3, in the latter. Consequently it was not surprising that the only male
hybrid whose chromosomes have been reported had 53 chromosomes (Cribiu
& Popescu, 1980). Since then, however, other animals studied in our
laboratory and coming from different institutions, have shown to possess
different karyotypes (2n=54-56). The problem with most of these animals
is that the precise place of origin of the animals studied is unknown.
Several hybrids of forest and "East African buffalo" ("S.
caffer aequinoctialis") were also produced at the Paris Zoo (Gray,
1972). It is unusual for a "good species" to have this wide
a range of karyotypes and more work with animals of known locality of
origin is needed. Moreover, it would be important to know whether putative
"hybrids" are fertile. The chromosome number of the specimen
examined here was 2n=55 (XX).
14)
Immunology
There are no reports of immunologic studies. Sporadic reports of the presence
of antibodies in buffalos have been published. Thus, Shepherd et al. (1987)
found antibodies against "Crimean-Congo hemorrhagic fever" in
56 of 287 specimens of African buffalo. In a later study (Burt et al.,
1993) expanded their study with modern antibodies and ascertained the
persistence of antibodies in sheep. The virus is transmitted by an adult
tick (Hyalomma) that is known to use buffalo as one of many hosts.
15)
Pathological features
It is believed that the savannah buffalo serves as reservoir of foot-and-mouth
disease in Africa (Vosloo et al., 2002). Syncerus also serves as
an important reservoir of Mycobacterium bovis (Grobler et al.,
2002). Attempts at removing infected animals are in progress. Nevertheless,
the disease is often spread to cattle and other domestic species and remains
to be a serious health problem (Michel, 2002). Huchzermeyer et al. (2001)
autopsied a number of animals, including forest buffalos, in the Congo
Republic. They determined that infection with Elaeophora sagitta
had caused exsanguinations, and exhaustion was finally caused by an "unusual
plague of Stomoxys omega". Griner (1983) recorded trauma,
enteritis and neonatal malnutrition from lack of maternal lactational
care as causes of deaths.
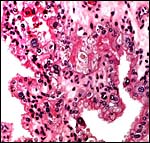
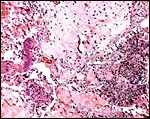
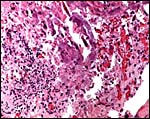
The placenta of the stillborn shown above was remarkable in that one half
was perfectly normal, with well-preserved cotyledons and showing no inflammation;
the darker cotyledons were massively congested and had large colonies
of bacteria in villi and there was acute inflammation associated with
these colonies, and located in the villous structures.
16)
Physiologic data
Pospisil et al. (1985) published the results of their hematologic studies
of both buffalo species. Red cell counts decreased with age, while the
percentage of eosinophils increased.
17) Other resources
Many cell lines of African buffalos are available from CRES
at the San Diego Zoo by contacting Dr. Oliver Ryder at oryder@ucsd.edu.
18)
Other remarks - What additional Information is needed?
This specimens were too autolyzed for a proper histological study. Because
of the thin, flat nature of cotyledons and the seemingly large areas of
connective tissue in the placenta, studies of implanted placentas is badly
needed. There is a need to better define the chromosomal features of animals
from know regions in Africa, known phenotypes, and of hybrids.
Acknowledgement
The animal photographs in this chapter come from the Zoological Society
of San Diego. I appreciate also very much the help of the pathologists
at the San Diego Zoo.
References
Atkinson, Y.H., Gogolin-Ewens, K.J., Hounsell, E.F., Davies, M.J., Brandon,
M.R. and Seamark, R.F.: Characterization of placentation-specific binucleate
cell glycoproteins possessing a novel carbohydrate. J. Biol. Chem. 268:26679-26685,
1993.
Burt, F.J., Swanepoel, R. and Braack, L.E.: Enzyme-linked immunosorbent assays for the detection of antibody to Crimean-Congo haemorrhagic fever virus in the sera of livestock and wild vertebrates. Epidemiol. Infect. 111:547-557, 1993.
Cribiu, E.P. and Popescu, C.P.: Chromosome constitution of a hybrid between East African buffalo (Syncerus caffer caffer) and dwarf forest buffalo (Syncerus caffer nanus) Ann Génét. Sél. Anim. 12:291-293, 1980.
Gray, A.P.: Mammalian Hybrids. A Check-list with Bibliography. 2nd edition. Commonwealth Agricultural Bureaux Farnham Royal, Slough, England, 1972.
Grimsdell, J.J.R.: Reproduction in the African buffalo, Syncerus cadder, in western Uganda. J. Reprod. Fertil. Suppl. 19:303-318, 1973.
Griner, L.A.: Pathology of Zoo Animals. Zoological Society of San Diego, San Diego, California, 1983.
Grobler, D.G., Michel, A.L., De Klerk, L.M. and Bengis, R.G.: The gamma-interferon test: its usefulness in a bovine tuberculosis survey in African buffaloes (Syncerus caffer) in the Kruger National Park. Onderstepoort J. Vet. Res. 69:221-227, 2002.
Groves, C.P.: Systematic relationships in the Bovini (Artiodactyla, Bovidae). Z. zool. Systematik Evol.fschg. 19:264-228, 1981.
Grubb, P.: Variation and incipient speciation in the African buffalo. Z. Säugetierk. 37:121-124, 1972.
Hsu, T.C. and Benirschke, K.: An Atlas of Mammalian Chromosomes. Vol. 4, Folio 192. Springer-Verlag, NY 1970.
Huchzermeyer, F.W., Penrith, M.L. and Elkan, P.W.: Multifactorial mortality in bongos and other wild ungulates in the north of the Congo Republic. Onderstepoort J. Vet. Res. 68:263-269, 2001.
Knechtel, C.: Brunstverhalten bei Kaffernbüffeln (Syncerus caffer caffer) im Tierpark Berlin-Friedrichsfelde. Der Zool. Garten 63:32-58, 1993.
Michel, A.L.: Implications of tuberculosis in African wildlife and livestock. Ann. N.Y. Acad. Sci. 969:251-255, 2002.
Mossman, H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Pohle, C.: Kaffernbüffel (Syncerus caffer caffer) im Tierpark - fast die letzten in deutschen Zoos. Milu10:616-624, 2002.
Pospisil, J., Kase, F. and Vahala, J.: Basic haematological values in the African buffalo (Syncerus caffer caffer) and in red buffalo (Syncerus caffer nanus). Comp. Biochem. Physiol. A 82:495-498, 1985.
Shepherd, A.J., Swanepoel, R., Shepherd, S.P., McGillivray, G.M. and Searle, L.A.: Antibody to Crimean-Congo hemorrhagic fever virus in wild mammals from Southern Africa. Amer. J. Trop. Med. Hyg. 36:133-142, 1987.
Simonsen, B.T., Siegismund, H.R. and Arctander, P.: Population structure of African buffalo inferred from mtDNA sequences and Microsatellite loci: high variation but low differentiation. Mol. Ecol. 7:225-237, 1998.
Sinclair, A.R.E.: The African Buffalo: A Study of Resource Limitation of Populations. Univ. Chicago Press, 1977.
Vosloo, W., Boshoff, K., Dwarka, R. and Bastos, A.: The possible role that buffalo played in the recent outbreaks of foot-and-mouth disease in South Africa. Ann. N.Y. Acad. Sci. 969:187-190, 2002.
Wild, A.: Untersuchungen über den Aufbau der Placenta fetalis des Rindes und ihre Auswirkungen auf die Gesundheit des Kalbes. Zentralbl. Veterinärmed. 11:60-89, 1964.
Wilson, D.E. and Reeder, D.A.M.: Mammal Species of the World. A Taxonomic and Geographic Reference. 2nd ed. Smithsonian Institution Press, Washington, DC, 1992.
Wooding, F.B.: The role of the binucleate cell in ruminant placental structure. J. Reprod. Fertil. Suppl. 31:31-39, 1982.
Wooding, F.B., Morgan, G. and Adam, C.L.: Structure and function in the ruminant synepitheliochorial placenta: central role of the trophoblast binucleate cell in deer. Microsc. Res. Tech. 38:88-99, 1997.
Wurster, D.H. and Benirschke, K.: Chromosome studies in the superfamily Bovoidea. Chromosoma 25:152-171, 1968.