| (Clicking
on the thumbnail images will launch a new window and a larger version
of the thumbnail.) |
| Last updated: March 17, 2004. |
Chinchilla laniger
With Peter Kaufmann
Order: Rodentia
Family: Chinchillidae
1) General Zoological Data
There are six species in this family of South American rodents, perhaps the most remarkable, from a reproductive point of view, is the viscacha. But there is also debate in the literature whether Chinchilla brevicauda should not be listed as a subspecies of the animal under discussion, rather than as a separate species. Indeed, whether chinchillas really "belong" to the hystricomorpha is debated and the reader is referred to the comprehensive symposium by Rowlands & Weir (1974) and the discussion already detailed in my chapter on Pacarana. Moreover, newer studies of mitochondrial RNA genes by Nedbal et al. (1995) suggested that there was a split in Eocene between Old and New World rodent groups and that caviomorphs are monophyletic. Thus, ongoing debate attempts to better understand the variety of South American rodents and their origin as well as taxonomic relations.
The name chinchilla is probably of Indian language origin; "laniger" refers to wool-bearing" (Gotch, 1979). The chinchilla, discussed here, Chinchilla laniger is now widely distributed through numerous chinchilla farms for the production of their delicate fur. Many color variations have been produced by breeders. In nature, the animal was primarily found in the Andes, often at high altitude and in barren areas. Very large numbers of animals formed colonies of up to 300 animals. Wild chinchillas are now rare and "protected", and reintroduction has been futile. Clark (1984) reported on the importation of chinchillas to California in 1923 and purported that "most commercially bred chinchillas are descended from these 11 animals". There are several good web sites (accessible through Google.com) on chinchillas, on their breeding requirements, feeding, gestation, their origin, and also on the original importer (M.P. Chapman), a mining engineer. The female chinchilla weighs up to around 800 g, males being slightly smaller. The longevity in captivity is 20 years (Nowak, 1999).
 |
Mature female chinchilla. |
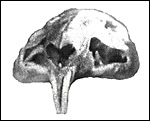 |
Skull of mature chinchilla with slightly yellow incisors. |
Chinchillas are polyestrous with a cycle varying between 35 and 60 days (Bronson, 1999) and they have a relatively long gestation of 110-130 days (mean 111 days - Weir, 1974). Two to three offspring are normal, but as many as six have been described. Neonates weigh 35 to 48 g; crown-rump length is 103 mm. Galton (1968) investigated the unusual sex ration of neonates (115:100). Placental weights are (at full term): 2 - 4 g and further available from the report of Roberts & Perry (1974). They gave a weight of the term chinchilla placental disk as 9 g, with a diameter of the disk as 2.2 cm.
3) Implantation
Implantation of the blastocyst occurs on day 5¼ after fertilization
and is on the antimesometrial side (Roberts, 1967). It was then found
to lie directly beneath the endometrial surface with remnants of parietal
trophoblast still visible. "By Day 8, elongation of the endoderm
has separated the amnioembryonic mass from the ectoplacental trophoblast
and, by Day 10, the latter has penetrated deep into the decidua. A small
slit in the amnioembryonic mass on Day 15 marks the beginning of amnion
formation and mesoblast lines the blastocyst. Between Days 20 and 30 the
allantois grows towards the chorion forming the chorioallantoic placenta."
While the zona pellucida is invaded early by trophoblast in chinchillas,
in most rodents, zonalysis occurs enzymatically (Abrahamson & Zorn,
1993). The mechanism in chinchillas has apparently not been studied in
greater detail.
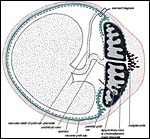
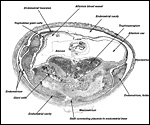
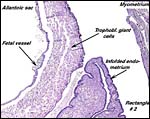
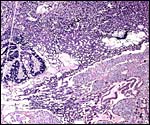
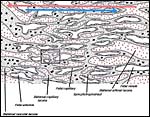
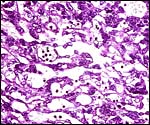
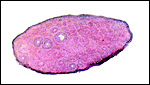
The chinchilla placenta is in many respects very similar to that of the guinea pig, and also to that of some other South American rodents. It is discoid, hemomonochorial, with a complex labyrinth, and it is "pedunculated" as will be seen in the low power views of an early gestation and an abortion specimen. It has a subplacenta and connects only through a narrow stalk with the decidualized endometrium (see Tibbitts & Hillemann, 1959; King & Tibbitts, 1976). It forms an inverted yolk sac relationship to the endometrium and possesses a thick layer of trophoblastic giant cells at the base that invade deeply into endo-myometrium. For the novice, not familiar with inverted yolk sac placentation, this is a complex configuration with the development of several sites for nutrients exchange. Reference is made to the chapters on mouse, rat, and pacaranas for additional details. For simplicity, however, a diagram is composed from the sections that follow.
Allantois: Only used for formation and vascularization of the chorioallantoic placenta (main placenta and subplacenta).
The chinchilla placenta has many similarities with the structure of the guinea pig placenta. The placental fine structure has been presented in exquisite detail by King & Tibbitts (1976). Their conclusion is that the chinchilla has a hemo-monochorial barrier in the large region of the lobulated "labyrinth". The trophoblast has numerous microvilli towards the maternal system and this syncytium has abundant granular endoplasmic reticulum. They found only few cytotrophoblastic cells beneath the syncytium of the labyrinth and these were then located above the delicate basement membrane. The trophospongium has trophoblast lining the maternal vascular channels. The yolk sac epithelium of the inverted yolk sac has columnar epithelium that borders on the markedly thinned endometrium of the antimesometrial sac. It, too, has numerous microvilli and pinocytotic vesicles testify to the absorptive nature of this region. It stains positive for iron as well.
Few characteristics are displayed by the large giant cells. Their large amount of cytoplasm has many vacuoles about whose possible content one is not altogether certain, other than that much of it is probably glycogen.
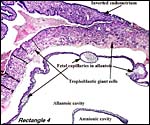
The exchange area, the labyrinth, is structurally identical with that of the guinea pig (King & Tibbitts, 1976) (next diagrams): the labyrinth is composed of finger-like lobes, each lobe representing an own circulatory unit: The maternal blood flows from a central arterial lacuna centrifugally via a web-like system of trophoblast-lined blood lacunae to the lobular periphery where it is drained by a web of venous lacunae. The fetal blood enters the same lobes via several fetal arterioles, located at the lobular surface; there they branch in to radially oriented capillaries which lead the fetal blood centripetally. It is collected by fetal venules in the lobar center. The blood flow arrangement is identical with that of all other caviomorph rodents and represents a perfect counter-current exchanger. The fetal/maternal barrier is generally hemomonochorial. Maternal and fetal bloods are separated by a layer of syncytiotrophoblast which is separated from the fetal endothelium in most places only by a basal lamina. Cytotrophoblast cells beneath the syncytiotrophoblast are rare; in larger numbers they were only found throughout the first weeks of pregnancy (King & Tibbitts, 1976).
This is divided, as in all rodents, into the portion supplying the inverted yolk sac, and the embryonic allantoic cord. Both are very short but measurements are not available and my sections did not happen to include the cords. I presume that each portion contains two vessels, as in pacarana.
7) Uteroplacental circulation
The distribution of the fetal vascular supply to the placental disk is
explained in some detail by Roberts & Perry (1974). The fetal vessels
enter centrally and radiate immediately and widely, thus supplying the
individual lobules of the labyrinth. A preceding photograph shows this
relationship. Maternal blood supply to the implantation sites is as described
for the guinea pig. At term, uteroplacental arteries show dilatation to
about 1mm luminal diameter not only in the uterine wall but centrally
exceeding for about 1 cm into the mesometrium. Though not proven in the
chinchilla, degree and location of arterial dilatation suggest that it
is caused by peritoneal trophoblast invasion as described for the guinea
pig (Nanaev et al., 1995).
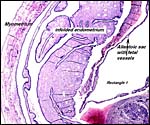
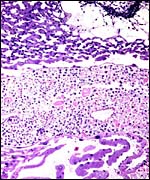
As in all other caviomorph rodents, also in the chinchilla the yolk sac placenta replaces the thin, non-placental, parts of the blastocystic trophoblast vesicle (King & Tibbitts, 1976). Together with the chorioallantoic placenta it forms the fetal membranes. Its endodermally covered and fetally vascularized folds are in intimate contact with the endometrial surface epithelium. There is a sizeable allantoic sac with a mesenchymal membrane exhibiting numerous fetal blood vessels that are filled with nucleated red blood cells in the specimen shown above. The yolk sac epithelium (opposite the endometrium) is cylindrical and has many pinocytotic vesicles. Active protein transport occurs at this site.
The amnion forms by cavitation and the amnionic epithelium is very thin and flat. Plaques were not present in my specimens and, in hystricomorphs, they have only been described in the viscacha (Roberts & Perry, 1974). There are no blood vessels in the amnion. The allantoic epithelium is cuboidal to cylindrical.
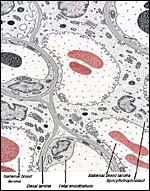
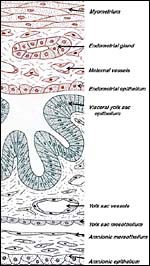 |
Schematics of visceral yolk sac placenta (green) of caviomorph rodent attached to uterine wall (red), and amnion (gray). |
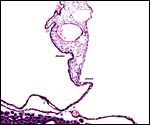 |
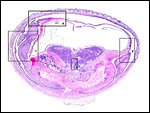
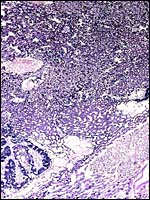
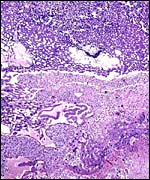
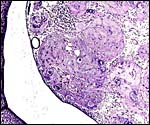
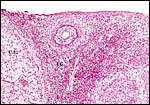
Allantoamnion in this immature placenta arising on the surface of the disk. |
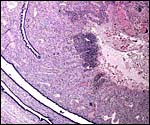
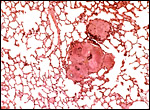
Aside from the deep invasion of the uterus by the trophoblastic giant cells, this species is peculiar in that many trophoblastic cells metastasize to the lung. This phenomenon of "adenoma" formation in the lung was first described in detail by Helmboldt et al. (1958) and it was then further examined in detail by Billington & Weir (1967). They found it to make protruding nodules in the lung that may persist up to 2 months after parturition, and even lasting into the next gestation. Mitoses were not present, and no such cells were found in uterine vein blood, unlike the finding in humans where this vascular deportation of syncytiotrophoblast is a regular feature of all pregnancies.
Tibbitts & Hillemann (1959) and King & Tibbitts (1976) described vacuolated giant cells in the junctional zone. It remained open, whether these are trophoblastic or decidual in nature. Own observations in the chinchilla suggest that trophoblast cells in this species are as invasive as in the guinea pig (Nanaev et al.,1995): both mononuclear trophoblast cells and multinucleated streamers of trophoblast spread from the subplacenta until deep into the junctional zone; mononucleated cells even pass the uterine wall and infiltrate the peritoneum. Both types of invasive trophoblast are embedded into massive accumulations of partly fibrous, partly glossy extracellular matrix which may well correspond to the matrix-type fibrinoid described as secretory product of invasive trophoblast in the human placenta (for review see Benirschke & Kaufmann, 2000).
The decidual transformation of rodent gestations, including the reduction of the decidual thickness at the antimesometrial pole, has been summarized in great detail in the study of Abrahamson & Zorn (1993). There is good decidual transformation of the endometrium and, in early stages, numerous mitoses may be found. In addition, one may identify scattered throughout some granulated metrial cells that have been discussed for rodent uteri in some detail by Peel (1989). She also referred to the rich and somewhat confusing literature on this topic. The then current view (for rats and mice) was that these cells are specialized "killer cells" that target trophoblast. Additionally it might be mentioned that in this placenta it is particularly easy, to identify Barr bodies in decidual cells, presumably because of the large X chromosome of these rodents.
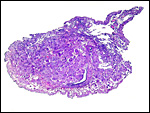
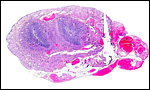 |
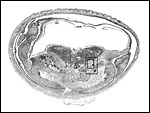
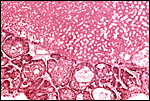
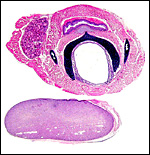
Bicornuate uterus of neonatal chinchilla. |
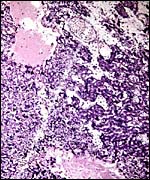
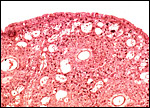
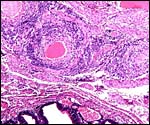
This species, as many other South American rodents, has a subplacenta. While it had initially been speculated that the "subplacenta" was a feature exhibited only by caviomorph rodents, subsequent studies have shown it to be present also in bathyergids, hystricids and thryonomids, according to Luckett (1980; see also Roberts & Perry, 1974). This region is thought to be a "growth zone for the proliferation of the trophoblast in the lobulated placental disc, and it has also been suggested as a possible site of placental endocrine activity". Maternal blood flow in these lacunae ceases already few weeks post conception. Fetal capillarization of the subplacenta starts only thereafter. Consequently, the subplacenta in no stage of pregnancy is simultaneously supplied by both, maternal and fetal blood flow and therefore cannot serve as an exchange organ. It degenerates a few days prior to parturition and leaves a mass of cellular detritus at the later site of placental separation.
In addition, the narrow "stalk" of the attachment site is characteristic of these rodents' placentas and it becomes quite attenuated at term gestation. This stalk comes about by "the fibrovascular ring which surrounds the site of attachment of the yolk sac splanchnopleure to the mesodermal body stalk at the fetal surface of the placenta. We have also observed an identical fibrovascular ring in hystricids and bathyergids. The functional significance of this structure remains unclear at present" (Luckett, 1980).
The necrotic basal portion beneath the disk (the "subplacenta") is due to extensive decidual necrosis and it contains occasionally maternal blood and many PAS-positive cells. It is especially extensive in chinchilla and viscacha (Roberts & Perry, 1974). Those authors considered that this region might produce the progesterone-binding globulin (PBG). King & Tibbitts (1976) also considered the secretory role of this region but hesitated to name a product.
12) Endocrinology
There is a large "interstitial gland" of the ovary that is already pronounced in neonates. Contrary to other hystricomorph rodents (see chapter on Pacarana), the ovaries of chinchillas are not folded and do not have a leaf-like surface. They are rather smooth and only the large interstitial gland is remarkable. Also unlike the viscacha, there is not the very large number of ovulation sites or corpora lutea. Weir suggested that the right ovary is more frequently employed. Under laboratory conditions chinchillas have a mean estrus cycle of 38 days. The females conceive easiest in a post-partum estrus starting 12 h after delivery and lasting 12 to 48 h, or in subsequent estrus periods (recognizable by reddish and swollen vulvas) starting 55 to 60 days after delivery.
The fetal adrenal has a wide "fetal cortex" but nothing is known of its product or possible involution after birth. Insulin receptors were studied immunologically in several species of rodents, including the chinchilla. These investigators found them to differ substantially from those of other mammals (Tong et al., 1994). Larsson et al. (1976) identified a "new hormone - PP" in the pancreas, including that of chinchillas.
Chinchillas have 64 chromosomes (all metacentrics) as was first determined by Nes (1963). One pair (# 2) has a secondary constriction and was considered to be a "marker chromosome". Galton et al. (1965) showed, with tritiated thymidine, the nature of the late replicating large X chromosome, and since then many other publications have verified the results of an unusually large X chromosome. Because of the large X chromosome of chinchillas (and other South American rodents), Johnson et al. (1987) were able to flow-sort the two populations of spermatozoa (X- and Y-bearing) very efficiently. Galton (1968) found a neonatal sex ratio of 119 M: 100 F and speculated that it might bear a relation to the disparity of sex chromosomal size, but had no other explanation for this unusual ratio. Gray (1972) listed hybrids between Chinchilla laniger and Chinchilla brevicauda as being fertile with progeny. Indirectly this supports the possible suspecific relationship rather than species rank of these two species.
 |
Male and female karyotypes with tritiated thymidine to show replication pattern. (From Hsu & Benirschke, 1967). |
Although it has been speculated that the metrial, granular cells of the endometrium may be a response to trophoblast, I am not aware of any study that affirms this conjecture. Also conjectural is the speculation that the deported trophoblast may be lysed by an immune mechanism. No details are available.
15) Pathological features
Griner (1983) found periodontal disease in chinchillas autopsied at San Diego Zoo. The animal has also been used for studies of Chagas' disease and for physiologic studies of hearing disorders because of its relative resistance to ear infections (Clark, 1984) and for the large size of the otic apparatus. Indeed, the entire ear has been of interest to otic investigator (see for instance: Mason et al., 2003). Giebink (1999) reviewed the topic of otitis media in chinchilla and declared that it is the only animal model in which pneumococcal otitis is easily studied and where it remains localized to the ear. Fungus infection of the skin and constipation are a frequent topic in the numerous excellent web sites on chinchillas (accessible via Google.com). Giardiasis with Giardia chinchillae is mentioned by Smith et al. (1972).
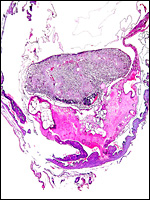 |
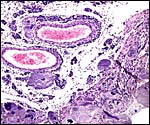
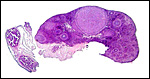
Uterus with aborted fetus and massive expansion of subplacenta. The narrow stalk to the decidua basalis is especially well displayed. |
Weir (1967) has detailed the problems of husbandry with chinchillas and discussed in some detail the aggressiveness of females. In their review of mammalian ovaries, Mossman & Duke (1973) wrote of a personal communication with Dr. B. Weir that indicated the development of new corpora lutea during gestation. There is an ovarian bursa with a wide opening, and the ovaries possess large interstitial glands. Weir (1970) described semen collection in chinchillas. Some other physiological data are listed in Alexander (!979) and in Clark's contribution (1984). Among other features, chinchillas have a high hemoglobin oxygen affinity (P50) (Ostojic et al., 2002).
Fine structural studies of the pineal gland undertaken by Matsushima & Reiter (1975) showed that, in contrast to other common rodents, the chinchilla pineal capillaries were lined by thick non-fenestrated endothelium.
17)
Other resources
We have no chinchilla cells in the CRES
cell bank of the San Diego Zoo.
18) Other remarks - What additional Information is needed?
Additional endocrine studies of pregnancy are warranted. Sections of umbilical
cords are needed.
References
Abrahamson, P.A. and Zorn, T.M.T.: Implantation and decidualization in
rodents. J. Exp. Zool. 266:603-628, 1998.
Alexander, N.J., ed.: Animal Models for Research on Contraception and Fertility. Harper & Row, Hagerstown, 1979.
Benirschke, K. and Kaufmann, P.: Pathology of the Human Placenta. Springer, New York, 2000.
Billington, W.D. and Weir, B.J.: Deportation of trophoblast in the chinchilla. J. Reprod. Fertil. 13:593-595, 1967.
Bronson, F.H.: Rodentia. In: Encyclopedia of Reproduction, E. Knobil & J.D. Neill, eds., Vol. 4, pp 282- 289, Academic Press, San Diego, 1999.
Clark, J.D.: Biology and Diseases of Other Rodents. Chapter 7 (pp. 184-206) in: Laboratory Animal Medicine. Fox, J.G., Cohen, B.J. and Loew, F.M., Academic Press Orlando, 1984.
Galton, M., Benirschke, K. and Ohno, S.: Sex chromosomes of the chinchilla: allocycly and duplication sequence in somatic cells and behavior of meiosis. Chromosoma 16:668-690, 1965.
Galton, M.: Chinchilla sex ratio. J. Reprod. Fertil. 16:211-216, 1968.
Giebink, G.S.: Otitis media: the chinchilla model. Microb. Drug Resist. 5:57-72, 1999.
Gotch, A.F.: Mammals - Their Latin Names Explained. Blandford Press, Poole, Dorset, 1979.
Gray, A.P.: Mammalian Hybrids. A Check-list
with Bibliography. 2nd edition.
Commonwealth Agricultural Bureaux Farnham Royal, Slough, England, 1972.
Griner, L.A.: Pathology of Zoo Animals. Zoological Society of San Diego, San Diego, California, 1983.
Hsu, T.C. and Benirschke, K.: An Atlas of Mammalian Chromosomes. Springer-Verlag, NY. Volume 1, Folio 19, 1967
Johnson, L.A., Flook, J.P., Look, M.V. and Pinkel, D.: Flow sorting of X and Y chromosome-bearing spermatozoa into two populations. Gamete Res. 16:1-9, 1987.
Kaufmann, P. and Davidoff, M.: The guinea-pig placenta. Adv. Anat. Embryol. Cell Biol. 53: 1-91, 1977.
King, B.F. and Tibbitts, F.D.: The fine structure of the chinchilla placenta. Amer. J. Anat. 145:33-56, 1976.
Larsson, L.I., Sundler, F. and Hakanson, R.: Pancreatic polypeptide - a postulated new hormone: identification of its cellular storage site by light and electron microscopic immunocytochemistry. Diabetologia 12:211-226, 1976.
Luckett, W.P.: Monophyletic or diphyletic origins of anthropoidea and hystricognathi. Evidence of the fetal membranes. Chapter 17, pp. 347-368, in: Evolutionary Biology of the New World Monkeys and Continental Drift. R.C. Ciochon & A.B. Chiarelli, Eds. Plenum press, N.Y., 1980.
Mason, K.M., Munson, R.S.Jr. and Bakaletz, L.O.: Nontypeable Haemophilus influenzae gene expression induced in vivo in a chinchilla model of otitis media. Infect. Immun. 71:3454-3462, 2003.
Matsushima, S. and Reiter, R.J.: Ultrastructural observations at pineal gland capillaries in four rodent species. Amer. J. Anat. 143:265-281, 1975.
Nanaev, A., Chwalisz, K., Frank, H.G., Kohnen, G., Hegele-Hartung, C. and Kaufmann, P.: Physiological dilatation of uteroplacental arteries in the guinea pig depends on nitric oxide synthase activity of extravillous trophoblast. Cell Tissue Res. 282:407-421, 1995.
Nedbal, M.A., Allard, M.W. and Honeycutt, R.L.: Molecular systematics
of hystricognath rodents: evidence from the mitochondrial 12S rRNA gene.
Mol. Phylogenet. Evol. 3:206-220, 1994.
Nes, N.: The chromosomes of Chinchilla laniger. Acta Vet. Scand. 4:128-135, 1963.
Nowak, R.M.: Walker's Mammals of the World. 6th ed. The Johns Hopkins Press, Baltimore, 1999.
Ostojic, H., Cifuentes, V. and Monge, C.: Hemoglobin affinity in Andean rodents. Biol. Res. 35:27-30, 2002.
Peel, S.: Granulated metrial cells. Advances Anat. Embryol. Cell Biol. 115:1-112, 1989.
Roberts, C.M.: The early development of some hystricomorph rodents with particular reference to Chinchilla laniger. J. Reprod. Fertil. 27:488-489, 1971.
Roberts, C.M. and Perry, J.S.: Hystricomorph biology and embryology, pp.333-360, in: The Biology of Hystricomorph Rodents. I.W. Rowlands and B.J. Weir, eds. Sympos. Zool. Soc. London, # 34. Academic Press, London, 1974.
Rowlands, I.W. and Weir, B.J.: The Biology
of Hystricomorph Rodents. Sympos. Zool. Soc. London, # 34. Academic Press,
London, 1974.
Smith, H.A., Jones, T.C. and Hunt, R.D.: Veterinary Pathology. Lea &
Febiger, Philadelphia, 1972.
Tibbitts, F.D. and Hillemann, H.H.: The development and histology of the chinchilla placentae. J. Morph. 105:317-365, 1959.
Tong, Z.Q., Jack, E., Moule, M., Goldfine, I.D. and Vip, C.C.: Rodent insulin receptors are immunologically different from other mammalian insulin receptors. Gen. Comp. Endocrinol. 94:374-381, 1994.
Weir, B.J.: The care and management of laboratory hystricomorph rodents. Lab. Anim. 1:95-104, 1967.
Weir, B.J.: Reproductive characteristics of hystricomorph rodents. Symp. Zool. Soc. London 34:265-301, 1974.
Weir, B.J.: Breeding techniques: Chinchillas. Pp. 209-223, in, Hafez E.S.E., ed.: Reproduction and Breeding Techniques for Laboratory Animals. Lea & Febiger, Philadelphia, 1970.