| 4 |
Tragulus javanicus
by Hiroaki Soma, Tokyo
Order: Artiodactyla
Family: Tragulidae
1) General Zoological Data
Tragulidae, chevrotains or mouse deer, comprise the water chevrotains of the genus Hyemoschus of Africa, the Indian spotted chevrotains of the genus Moschiola and the Asiatic chevrotains of the genus Tragulus. They represent an ancient group of ruminant species (Chikuni et al., 1995; Vrba & Schaller, 2000). The latter genus has two species (T. javanicus and T. napu) (Nowak, 1999). T. napu is depicted in the summary of San Diego Zoo's historical mammal description (Dolan & Killmar, 1989). The derivation of the words is as follows: "tragulus" (Gr. = goat). The word chevrotain derives from French: chèvre = a female goat (Gotch, 1979). The Malay chevrotain is the smallest artiodactyl described, weighing up to 8 kg, but more often it is 2.5-4.5 kg. Upper canines are strongly developed and they may live in captivity up to 16+ years (Jones, 1993). They are secretive, grazing animals and endangered species because of loss of habitat.
 |
Tragulus javanicus in Tokyo Zoo. |
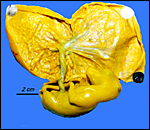
Gestational length is described to be from 140 to 177 days, with usually a single young resulting that weighs around 375 g at birth. The specimen available comes from a pregnant animal that died in mid-pregnancy. The uterus was fixed in Bouin's solution, which is the reason for the yellow stain but which also provides the excellent fixation. The uterus measured 15 x 9 cm and contained a female fetus with a 7 cm CR length.
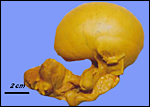 |
Uterus containing placenta and fetus. |
Implantation is shallow and occurs over both uterine horns. No cotyledons are formed as they are in other deer. Early stages have not been observed and timing of implantation is so far unknown. A single pregnant uterus of a mid-gestation was available for study. A most detailed study of the placentation of this species has recently been published by Kimura et al. (2004) whose study relied on nine specimens.
In contrast to other deer, this animal does not possess placental cotyledons; it also shows no real villous structures, at least at this young age. The placenta is more similar to that of camels and pigs and was so described already by Turner (1876) and Garrod & Turner (1878). It is a more or less diffuse epitheliochorial placenta that extended over both uterine horns. Concave protuberances could be palpated on the fetal surface of the placenta.
5) Details of fetal/maternal barrier
This is a typical epitheliochorial placenta without trophoblast infiltration of the endometrium. The trophoblast stained strongly with cytokeratin and weakly with placental alkaline phosphatase (PALP) reactions. Binucleate trophoblastic cells are abundant and stained positively with antibodies against bovine placental lactogen. They also stained strongly with PAS. Fetal and maternal vessels stained strongly with vimentin.
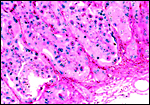 |
Implanted placenta with endometrium bottom right. |
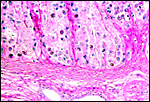 |
Implantation site with endometrial glands below. |
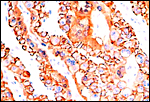 |
Cytokeratin staining of trophoblast. |
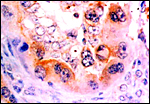 |
Binucleate cells stained with anti-bovine lactogen. |
The umbilical cord of this small fetus was 4 cm long and had 4 blood vessels in addition to the allantoic duct. It was also slightly spiraled.
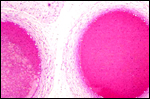 |
Section of two vessels in the umbilical cord and edge of allantoic duct (above). |
These structures have not been studied in detail.
8) Extraplacental membranes
There is an elongated allantoic sac containing sparse blood vessels. The amnion has a single layer of squamous epithelium.
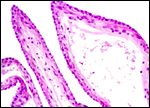 |
Allantoic membrane of chevrotain. |
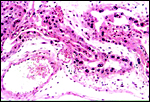 |
Chorionic membrane forming the arcades. Note also the binucleate cells. |
No trophoblastic infiltration of endometrium or vessels occurs.
10) Endometrium
There is no decidua.
11) Various features
None.
12) Endocrinology
I am not aware of many endocrine studies having been done in this species.
The presence of binucleate cells with positive reaction to bovine placental
lactogen is in conformity with other ruminants (see Atkinson et al., 1993,
and Wooding, 1982). The summary of deer endocrinology by Plotka (1999)
does not include this species. Wallis & Wallis (2001) described the
evolution of growth hormone in this species.
The animals have 32 chromosomes with a large X-chromosome as shown below (Soma, unpublished; Gallagher et al., 1996; Liming & Yuze, 1989; Yong, 1973). The same findings were made in T. javanicus williamsoni. Hybrids have not been described. New molecular data that show the ancient origin of this Order, including lesser and larger mouse deer, have been provided by Chikuni et al. (1995). Cytochrome c gene sequences were studied by Irwin et al. (1991). Breukelman et al. (2001) discussed the phylogenetic sequence by studying secretory ribonucleases (RNases).
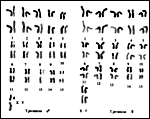 |
Karyotypes of Malay chevrotains. |
We are not aware of any relevant studies.
15) Pathological features
The only abnormal findings made in Malayan chevrotains were malnutrition
(Griner, 1983), and in an African chevrotain Griner found glomerulosclerosis.
Grondahl et al. (2003) found bovine diarrhea viruses in a Malay mouse
deer. Ciliated parasites (Isotrichia jalaludinii) were identified
in the stomach of lesser mouse deer by Imai et al. (1995).
16) Physiologic data
Mossman & Duke (1973) described a large thecal ovarian gland in mouse
deer. These animals also possess the smallest erythrocytes of mammalian
species. They are spherical and thus cause increased viscosity of blood
despite which cardiac output etc. are within normal limits (Weathers &
Snyder, 1977). Unique pits were observed in the red blood cells by Fukuta
et al. (1996), using scanning EM. Electroejaculation and the semen obtained
were published by Haron et al. (2000).
17)
Other resources
Some fibroblast cell strains are available from CRES
at the San Diego Zoo by contacting Dr. Oliver Ryder at oryder@ucsd.edu.
18) Other remarks - What additional
Information is needed?
Early stages of implantation and measurements of term placentas and umbilical
cord are needed.
Acknowledgement
The animal photograph in this chapter comes from Professor Hiroaki Soma.
I am sincerely grateful for his contributing this placenta to this site
(KB).
Tragulus javanicus in Tokyo Zoo. This animal was provided by the Malay
Breeding Center, courtesy of Associate Prof. Junpei Kimura, Veterinary
Department, Nihon University, Fujisawa, Kanagawa, Japan. Accordingly,
I would like to acknowledge his kind cooperation.
References
Atkinson, Y.H., Gogolin-Ewens, K.J., Hounsell, E.F., Davies, M.J., Brandon,
M.R. and Seamark, R.F.: Characterization of placentation-specific binucleate
cell glycoproteins possessing a novel carbohydrate. J. Biol. Chem. 35:26679-26685,
1993.
Breukelman, H.J., Jekel, P.A., Dubois, J.Y., Mulder, P.P., Warmels, H.W. and Beintema, J.J.: Secretory ribonucleases in the primitive ruminant chevrotain (Tragulus javanicus). Eur. J. Biochem. 268:3890-3897, 2001.
Chikuni, K., Mori, Y., Tabata, T., Saito, M., Monma, M. and Kosugiyama, M.: Molecular phylogeny based on the k-casein and cytochrome b sequences in the Mammalian Suborder Ruminantia. J. Mol. Evol. 41:859-866, 1995.
Dolan, J.M. and Killmar, L.E.: The Mammal Collection of the Zoological Society of San Diego, a historical perspective. Zool. Garten 59:77-121, 1989.
Fukuta, K., Kudo, H. and Jajaludin, S.: Unique pits on the erythrocytes of the lesser mouse-deer, Tragulus javanicus. J. Anat. 189:211-213, 1996.
Gallagher, D.S., Houck, A.M., Ryan, J.E., Womack, J.E. and Kumamoto, A.T.: A karyotypic analysis of the lesser Malay chevrotain, Tragulus javanicus (Artiodactyla:Tragulidae). Chromosome Res. 4:545-551, 1996.
Garrod, A.H. and Turner, W.: On the gravid uterus and placenta of Hyomeschus aquaticus. Proc. London Zool. Soc. 14:682-686, 1878.
Gotch, A.F.: Mammals - Their Latin Names Explained. Blandford Press, Poole, Dorset, 1979.
Griner, L.A.: Pathology of Zoo Animals. Zoological Society of San Diego, San Diego, California, 1983.
Grondahl, C., Uttenthal, A., Houe, H. Rasmussen, T.B., Hoyer, M.J. and Larsen, L.E.: Characterisation of a pestivirus isolated from persistently infected mouse deer (Tragulus javanicus). Arch. Virol. 148:1455-1463, 2003.
Haron, A.W., Ming, Y. and Zainuddin, Z.Z.: Evaluation of semen collected by electroejaculation from captive lesser Malay chevrotain (Tragulus javanicus). J. Zoo Wildl. Med. 31:164-167, 2000.
Imai, S., Kudo, H., Fukuta, K., Abudullah, N., Ho, Y.W. and Onodera, R.: Isotricha jalaludinii n. sp. found from the rumen of lesser mouse deer, Tragulus javanicus, in Malaysia. J. Eukaryot. Microbiol. 42:75-77, 1995.
Irwin, D.M., Kocher, T.D. and Wilson, A.C.: Evolution of the cytochrome b gene of mammals. J. Mol. Evol. 32:128-144, 1991.
Jones, M.L.: Longevity of ungulates in captivity. Intern. Zoo Yearbk. 32:159-169, 1993.
Kimura, J., Sasaki, M., Endo, H. and Fukuta, K.: Anatomical and histological characterization of the female reproductive organs of mouse deer (Tragulidae). Placenta 25:705-711, 2004.
Liming Shi and Yuze Chen: The karyotype analysis of Yunnan mouse deer (Tragulus javanicus williamsoni). Acta Zool. Sinica 35:41-43, 1989. (In Chinese).
Mossman, H.W. and Duke, K.L.: Comparative Morphology of the Mammalian Ovary. University of Wisconsin Press, Madison, Wisconsin, 1973.
Nowak, R.M.: Walker's Mammals of the World. 6th ed. The Johns Hopkins Press, Baltimore, 1999.
Plotka, E.D.: Deer. Pp. 842-857, in Encyclopedia of Reproduction, Vol. 1, E. Knobil and J.D. Neill, eds. Academic Press, San Diego, 1998.
Strahl, H.: Zur Kenntnis der Placenta von Tragulus javanicus. Anat. Anz. 26:425-428, 1905.
Turner, W.: On the structure of the diffused, the polycotyledonary and zonary forms of placenta. J. Anat. Physiol. 10:127-177, 1876.
Vrba, E.D. and Schaller, G.B.: Antelopes, Deer, and Relatives. Fossil Record, Behavioral Ecology, Systematics, and Conservation. Yale University Press, New Haven and London, 2000.
Wallis, O.C. and Wallis, M.: Molecular evolution of growth hormone (GH) in Certartiodactyla: cloning and characterization of the gene encoding GH from a primitive ruminant, the chevrotain (Tragulus javanicus). Gen. Comp. Endocrinol. 123:62-72, 2001.
Weathers, W.W. and Snyder, G.K.: Hemodynamics of the lesser mouse deer, Tragulus javanicus. J. Appl. Physiol. 42:679-681, 1977.
Wooding, F.B.: The role of the binucleate cell in ruminant placental structure. J. Reprod. Fertil. Suppl. 31:31-39, 1982.
Yong, H.S.: Complete Robertsonian fusion in the Malaysian lesser mouse-deer (Tragulus javanicus). Experientia19:366-367, 1973.