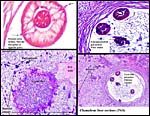
| (Clicking
on the thumbnail images will launch a new window and a larger version
of the thumbnail.) |
| Last updated: Nov 30, 2006. |
Chamaeleon jacksonii xantholophus
Class: Reptilia
Order: Squamata
Family: Chamaeleonidae
1) General Zoological Data
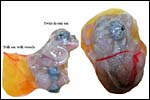
While chameleons are widely distributed over the globe, this species originated in the highlands of Kenya/Tanzania and was introduced into Hawaii in 1972 where it is now abundant and locally destructive (see G.H. Waring, internet). Abate (internet reference) stated that the fossil appearance of chameleons is about 60 Million years, but the oldest fossil identified securely is 26 million years old. Adult male Jackson 's chameleons possess 3 horns (two preorbital and one rostral) and are therefore referred to as ‘three-horned chameleons' in the German literature. Females are smaller and have no horns. This is a primarily arboreal species whose adults are from 152-711 mm in length, nearly one-half made up of the tail. Their longevity is variably given as up to 8-11 years in captivity. Of course, chameleons are best known for their ability of changing color and for their large protruding and independently moving eyes. The large eyes are readily seen in the photographs. Approximately 20% of squamates are viviparous, as is this species ( Blackburn , 1998). The developing placenta represents an interesting and variably constructed transition to mammalian placentation and has thus attracted much attention.
The Chamaeleonidae comprise 6 genera with around 180 species and subspecies (Abate – internet reference), and there is also a wild, introduced population in California , in addition to that in several islands of Hawaii . A large number of web sites provide information regarding their care, nutrition, diseases and supplies needed for maintenance.
 |
Adult male Jackson 's chameleon (from the web sites). |
 |
Adult female chameleon (from the web sites). |
2) General Gestational Data
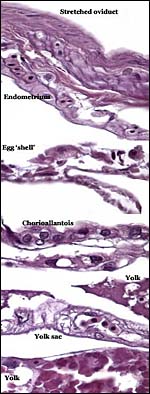
Jackson's chameleon is ‘ovoviviparous' (others have referred to the same feature as being viviparous), i.e. the female carries her young to maturity and, when the young are born, their thin enclosing membrane ruptures immediately and the offspring become completely independent of maternal care, consuming some of the membranes. Daan (1971) described that from 36-40 embryos are gestated whose measurement is 52-55 mm at birth with a 23-25 mm length of the tail. They begin catching flies immediately and grow rapidly so that, at 5 months of age, they measure 8-10 cm. While only 20% of squamates are viviparous, their developing membranes are of great interest to evolutionists (see Blackburn et al., 1984; Blackburn , 1992). There are species that deposit their eggs and are completely lecithrotrophic (from lekithos – Gr. = yolk ), to other forms with partial matrotrophy and chorioallantoic placentation. Stewart (1992) reflected on the different origins of nutrients, gases etc. from yolk and through exchange via the chorioallantoic vascular system with the oviduct; he also showed the mesodermal ‘invasion' of the yolk.
3) Implantation
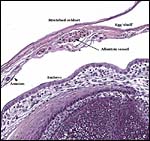
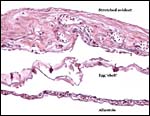
Early implantational stages have apparently not been published for this species, nor has the development of the ‘membranes' been discussed let alone studied in detail. Stewart & Blackburn (1988) have discussed at length the terminology to be used for reptilian placental membranes and, so far as this animal's gestation is concerned, the term “chorioallantoic placenta” best describes the membranes. Gestation is described as being 5-9 months long (see the Internet: Answers.com).
4) General Characterization of the Placenta
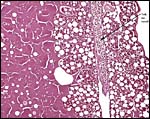
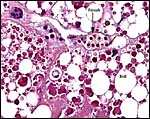
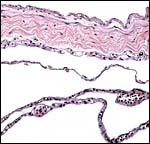
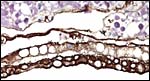
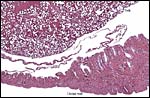
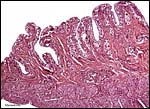
The structure of reptilian placentas has been reviewed and studied numerous times and by many different investigators (see for instance: Wooding & Flint, 1994 and the precursors to the “ Marshall ”). They are of interest because they foreshadow the development of the derived mammalian organ; they also differ in complexity and function, as well as in their surface apposition to the maternal epithelium of the oviduct or uterus. In most review articles it is stated that doubtless the type or pre-mammalian placentation of the reptiles has been ‘invented' independently several times during evolution. The placenta to be considered here then is a chorioallantoic organ with a prominent yolk sac from which early nutrition is obtained. What are of special interest though are the numerous vitelline vessels that traverse the yolk and surround it as well. In addition to the yolk sac nourishment, doubtless through the chorioallantoic approximation to the maternal system, fluid and gases at a minimum may be obtained from the uterine circulation. Stewart (1992) suggested that much calcium may also be thus obtained, despite the fact that yolk has much calcium content; how Chameleons do it is unknown as yet. Several authors have alluded to their finding that the viviparous reptilian placenta interdigitates with the oviductal epithelium and is thus difficult to dislodge, without, however, invading it. While the conceptus may be difficult to dislodge because of the interdigitation, no histologic suggestions for these mesenchymal interdigitations were evident in this specimen.
The numerous contributions by Blackburn and Stewart to the understanding of reptilian placentation are especially noteworthy. In 2003, Stewart & Thompson described the different types of placental development of different reptiles and enumerated their complexities; they insisted that the different types evolved at different times and that they did this repeatedly so. For instance, in their presentation on Niveoscincus ocellatus (Stewart & Thompson, 2004), they found an omphaloplacenta with thin intervening ‘inter-omphalopleuric' membrane and placentome and make more reference to the concept.
Blackburn et al. (2003) examined the question as to the possible resorption of dead embryos in Pseudemoia pagenstecheri at different stages of gestation. While a number of dead embryos were found, and some had the appearance of being moved along the oviduct, no true resorption could be identified.
5) Details of the fetal/maternal barrier
The fetal-maternal relation is always epithelio-chorial. In some reptilian species binucleated trophoblastic cells have been described; I found none in this species. In fact, the trophoblast was very inapparent and, despite excellent fixation, difficult to clearly define. It was cytokeratin-positive.
6) Umbilical cord
There is no true umbilical cord.
7) Uteroplacental circulation
This has not been studied in this species but Amoroso (1961) has considered in some detail the various capillary proximities identified in different reptilian placentas.
8) Extraplacental membranes
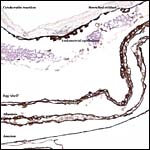
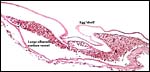
The membrane relationships between placenta and uterus have been dealt with in some excellent diagrammatic pictures presented by Weekes (1935). In addition to the chorioallantoic membranes to be depicted in several photographs below, there was an amorphous and brittle egg membrane (‘shell').
9) Trophoblast external to barrier
There is never any ‘invasion' of the oviduct by trophoblast, but the placenta is attached to the endometrium by some connective tissue.
10) Endometrium
In her final review article on reptilian placentation, Weekes (1935) described in considerable detail the oviductal/uterine structure of oviparous and viviparous reptiles, as well as their blood supply. She divided the oviduct into three different and functionally diverse structures. Girling (2002) has extensively reviewed the physiology, structure, and the needs for future research on all aspects of the reptilian oviduct and its physiological control. A true decidua is not produced but the epithelium subservices numerous important functions.
11) Various features
Numerous other details of reptilian reproduction could be discussed here, but they require more space than is available.
12) Endocrinology
Porter et al. (1982) have discussed in some considerable detail the evolution of placentation from its precursors to that of mammals and, especially, the manufacture and importance of hormones in different classes of animals. Of interest thus is the occurrence of LH-like peptides in the pituitary of birds and chelonia. The importance of progesterone production was equally well covered by these investigators. In some reptiles with chorioallantoic placenta, studies of the steroid metabolism have been performed. Thus, Painter & Moore (2005) incubated fetal membranes of the viviparous lizard Sceloporus jarrovi , and found that progesterone and corticosterone were metabolized to ‘other products'. The placenta was also able to synthesize progesterone from pregnenolone. Guarino et al. (1998) examined the endocrine activity of the viviparous skink Chalcides chalcides , a species often studied by other physiologists. Its corpus luteum was ‘compact', without internal vascularized septa and began to degenerate half-way through gestation when plasma progesterone levels were high. In contrast to the CL, the placenta always gave a ‘negative 3ß-HSDH reaction' in early and mid-pregnancy, while the reaction was strongly positive in late pregnancy and localized to the ‘maternal component of the placenta'. The addition of pregnenolone enhanced progesterone production, while its inhibitor (trilostane) decreased it.
13) Genetics
This species possesses 24 chromosomes, 4 of which are microchromosomes (Gorman, 1973). There is no sex chromosomal heteromorphism.
14) Immunology
Unfortunately, I was unable to identify physiological studies in Jackson 's chameleon reproduction, but physiologists such as Romagnoli et al. (2003) have shown that the skink placenta produces interleukin-1 and that its receptors are found in the oviductal epithelium during the peri-implantation period and suggested that it may be needed for the implantation/recognition process of the conceptus. In a later study from the same laboratory, Paulesu et al. (2005) showed that a species using oviparous, and others with ovoviviparous development (the lizard Lacerta vivipara ), that Il-1 and its receptor are present irrespective of the developmental process. This suggested to these investigators that the presence of these cytokines was not an essential condition for the acceptance of the conceptus by the oviduct. More interesting still is the study by Paulesu et al. (2001) on the Hß58 gene. Disruption of this complex in mice leads to reabsorption; it was thus of interest to learn of its strong expression in the skink Chalcides chalcides , both in the endometrium and the cytoplasm of the placental chorioallantoic epithelium. Moreover, this gene has a high degree of sequence homology with that of the rat. These authors also found increases in expression during gestation and, as in rodents, they speculated on the need for normalcy of this construct for placental development.
15) Pathological features
Griner (1983) has summarized his experience with various reptiles coming to autopsy at the San Diego Zoo. It included some chameleons from Madagascar and various parasites and infectious causes were listed as causes of death. An important category was ‘malnutrition', as the maintenance of some chameleons can be quite difficult. There were no neoplasms in his findings. Reportedly, parasitic infestation (nematodes) and protozoan infections are common findings in dead chameleons. The origin of the terminal septicemia with gram-negative organisms in this animal discussed here was not identified. Vitamin A deficiency, rickets due to inadequate UV light, respiratory diseases, tongue problems and ‘mouth rot' have all been discussed extensively in web sites. “Stress” is often mentioned as well as a cause of inappetance and disease. Occasional polydactyly has also been reported and it may be hereditary. Gartrell et al. (2004) reported a case of disseminated mycosis in a male chameleon that succumbed with anorexia and orbital swelling.
16) Physiologic data
Aside from the fascinating aspect of prey prehension and catching prey, much research has been done on the unusual mobility of the tongue; these considerations are well reviewed by Abate (internet). Similarly, Blackburn (1998) and Stewart (1992) discuss at some length the acquisition of water and anions (calcium, sodium) by the developing fetus.
17) Other resources
I am not aware of any other resources for chameleons, except to say that they are now abundant in Hawaii and California and can be purchased legally.
18) Other remarks – What additional Information is needed?
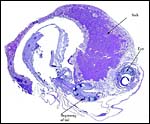
Certainly early implantational stages and electronmicroscopy of the placental interface would be of interest. For comparison, I next show a diagram from the literature on a viviparous reptilian placentation and, finally, the arrangement of fetus, yolk and membranes in the chicken.
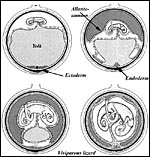 |
Schematic development of a viviparous lizard. From, Mossman (1987). |
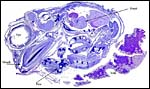
| Arrangement of developing embryo in the chicken. | |
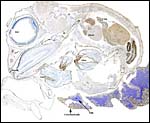
| Here follow some additional sections of embryos and membranes. | |
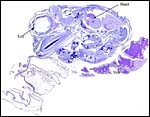 |
|
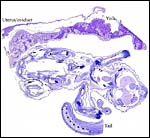 |
|
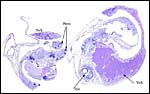 |
|
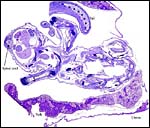 |
|
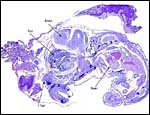 |
|
Acknowledgement
The animal photographs in this chapter come from the web sites; the specimen was collected by friends at the Threeringranch.org in Hawaii .
References
Abate, A.L.: Chameleons (Chamaeleonidae), in Internet Site: “Answers.com” www.answers.com/topic/chameleons-chamaeleonidae-biologial .
Amoroso, E.C.: Placentation. Chapter 15, pp. 127-311, in A.S. Parks, ed., Marshall's Physiology of Reproduction, Vol. II, 1961. Little, Brown and Company, Boston .
Blackburn , D.G.: Convergent evolution of viviparity, matrotrophy, and specializations for fetal nutrition in reptiles and other vertebrates. Amer. Zool. 32:313-321, 1992.
Blackburn , D.G.: Placenta and Placental Analogs in Reptiles and Amphibians. Pp. 840-847, Vol. 3, in, E. Knobil and J.D. Neill, eds.: Encyclopedia of Reproduction. Academic Press, San Diego , 1998.
Blackburn , D.G.: Reconstructing the evolution of viviparity and placentation. J. Theoret. Biol. 192:183-190, 1998.
Blackburn , D.G., Vitt, L.J. and Beuchat , C.A. : Eutherian-like reproductive specializations in a viviparous reptile. Proc. Natl. Acad. Sci. 81:4860-4863, 1984.
Blackburn , D.G., Weaber, K.K., Stewart, J.R. and Thompson, M.B.: Do pregnant lizards resorb or abort inviable eggs and embryos? Morphological evidence from an Australian skink, Pseudemoia pagenstecheri . J. Morphol. 256:219-234, 2003.
Daan, S.: Agamen und Chamäleons. Pp. 207-245, chapter 9 in Grzimeks Tierleben, Kindler Verlag, Zürich, 1971.
Gartrell, B.D., Alley, M.R. and Geschke, K.: Disseminated mycosis in a Jackson 's chameleon (Chamaeleo jacksonii). New Zeal. Vet. J. 52:51, 2004.
Girling, J.E.: The reptilian oviduct: a review of structure and function and directions for future research. J. Exp. Zool. 293:141-170, 2002.
Gorman, G.C.: Chromosomes of reptilian. Chapter 12, pp. 349-424, in A.B. Chiarelli & E. Capanna: Cytotaxonomy and Vertebrate Evolution. Academic Press, London , 1973.
Griner, L.A. : Pathology of Zoo Animals. Zoological Society of San Diego , San Diego, California, 1983.
Guarino, F.M., Paulesu, L., Cardone, A., Bellini, L., Ghiara, G. and Angelini, F.: Endocrine activity of the corpus luteum and placenta during pregnancy in Chalcides chalcides (Reptilia, Squamata). Gen. Comp. Endocrinol. 111:261-270, 1998.
Mossman, H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Painter, D.L. and Moore, M.C.: Steroid metabolism by the chorioallantoic placenta of the Mountain Spiny Lizard Sceloporus jarrovi as a possible mechanism for buffering maternal-fetal hormone exchange. Physiol. Biochem. Zool. 78:364-372, 2005.
Paulesu, L., Cateni, C., Romagnoli, R., Chellini, F., Angelini, F., Guarino, F.M., Rider, V., Imakawa, K. and Bigliardi, E.: Evidence of Hß58, a gene involved in mammalian placental development, in the three-toed skink, Chalcides chalcides (Squamata: Scincidae), a viviparous placentotrophic reptile. Placenta 22:735-741, 2001.
Paulesu, L., Bigliardi, E., Paccagnini, E., Letta, F., Cateni, C., Guillaume, C.P. and Heulin, B.: Cytokines in the oviparity/viviparity transition: evidence of the interleukin-1 system in a species with reproductive bimodality, the lizard Lacerta vivipara . Evol. Devel. 7:282-288, 2005.
Porter, D.G., Heap, R.B. and Flint , A.P.F.: Endocrinology of the placenta and the evolution of viviparity. J. Reprod. Fertil. Suppl. 31.113-138, 1982.
Romagnoli, R., Cateni, C., Guarino, F.M., Bigliardi, E. and Paulesu, L.R.: Potential role of onterleukin-1 at the peri-ovulation stage in a species of placental viviparous reptile, the three-toed skink, Chalcides chalcides (squamata: scincidae). Reprod. Biol. Endocrinol.1:60, 2003.
Stewart, J.R.: Placental structure and nutritional provision to embryos in predominantly lecithotrophic viviparous reptiles. Amer. Zool. 32:303-312, 1992.
Stewart, J.R. and Blackburn , D.G.: Reptilian placentation: Structural diversity and terminology. Copeia 1988 (4):839-852.
Stewart, J.R. and Thompson, M.B.: Evolutionary transformations of the fetal membranes of viviparous reptiles: a case study of two lineages. J. Exp. Zool. A. Comp. Exp. Biol. 299:13-32, 2003.
Stewart, J.R. and Thompson, M.B.: Placental ontogeny of the Tasmanian scincid lizard, Niveoscincus ocellatus (Reptilia: Squamata). J. Morphol. 259:214-237, 2004.
Waring, G.H.: Preliminary study of the behavior and ecology of Jackson 's chameleons of Maui, Hawaii. http://www.hear.org/AlienSpeciesInHawaii/waringreports/chameleon.htm .
Weekes, C.: On Placentation in Reptiles No. i. 1. Denisonia superba and D. suta; 2. Lygosoma (Liolepisma) weekesae. Proc. Linnean Soc. N.S.W. 52:25-32, 1927.
Weekes, C.: A review of placentation among reptiles with particular regard to the function and evolution of the placenta. Proc. Zool. Soc. London 2:625-645, 1935.
Wooding, F.B.P. and Flint, A.P.F.: Placentation, pp. 235-460, in: G.E. Lamming, ed. Marshall's Physiology of Reproduction, 4 th edition, Volume 3, Chapman & Hall, London, 1994.