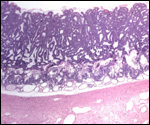
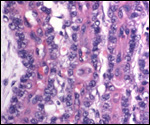
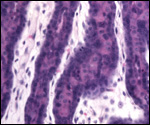
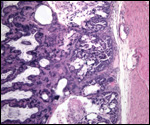
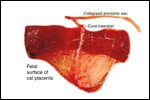
| |
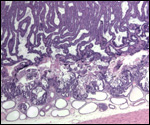
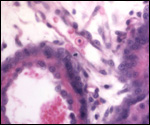
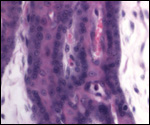
16) Physiological data
I do not know any relevant data.
17) Other resources
Cell lines are available from the "Frozen Zoo" of CRES at the
Zoological Society of San Diego. Reinert & Smith (1966) described
the establishment of an experimental cat breeding colony.
References
Baker,
H.J., Lindsey, J.R., McKhann, G.M. and Farrell, D.F.: Neuronal GM1 gangliosidosis
in a Siamese cat with beta-galactosidase deficiency. Science 174:838-839,
1971.
Benirschke,
K., Garner, F.M. and Jones, T.C.: Pathology of Laboratory Animals. 2 Vol.
Springer-Verlag, New York, 1978.
Benirschke,
K., Edwards, R. and Low, R.J.: Trisomy in a feline fetus. Amer. J. Vet.
Res. 35:257-259, 1974.
Björkman,
N.: Fine structure of the fetal-maternal area of exchange in the epitheliochorial
and endotheliochorial types of placentation. Acta anat. 86 (Suppl. 1):1-22,
1973.
Brown, J.L., Graham, L.H., Wu, J.M., Collins, D. and Swanson, W.F.: Reproductive
endocrine responses to photoperiod and exogenous gonadotropins in the
Pallas' cat (Otocolobus manul). Zoo Biol. 21:347-364, 2002.
Centerwall,
W.R. and Benirschke, K.: An animal model for the XXY Klinefelter syndrome
in man: Tortoiseshell and calico male cats. Amer. J. Vet. Res. 36:1275-1280,
1975.
Cho,
K.-W., Satoh, H., Youn, H.-Y., Watari, T., Tsujimoto, H., O'Brien, S.J.
and Hasegawa, A.: Assignment1 of the cat immunoglobin heavy chain genes
IGHM and IGHG to chromosomes B3q26 and T cell receptor chain gene TCRG
to A2q12-q13 by fluorescence in situ hybridization. Cytogenet. Cell Genet.
79:118-120, 1997.
Cho, K.-W., Satoh, H., Youn, H.-Y., Watari, T., Tsujimoto, H. and Hasegawa,
A.: Assignment of the feline c-mycgene (MYC) to cat chromosome F2q21.2
by fluorescence in situ hybridization. Cytogenet. Cell Genet. 78:135-136,
1997.
Cho,
K.-W., Okuda, M., Endo, Y., Satoh, H., Kang, C.-B., Watari, T., Tsujimoto,
H. and Hasegawa, A.: Assignment1 of the cat p53 tumor suppressor gene
(TP53) to cat chromosome E1p14-p13 by fluorescence in situ hybridization.
Cytogenet. Cell Genet. 79:145-146, 1997.
Cho,
K.-W., Tsujimoto, H., Hasegawa, A. and Satoh, H.: The cat immunoglobulin
lambda light chain gene maps to chromosome D3p12-p11. Mammal. Genome 9:178-179,
1997.
Chu,
E.H.Y., Thuline, H.C. and Norby, D.E.: Triploid-diploid chimerism in a
male tortoiseshell cat. Cytogenet. 3:1-18, 1964.
Collier,
G.E. and O'Brien, S.J.: A molecular phylogeny of the felidae: Immunological
distance. Evolution 39:473-487, 1987.
Dempsey,
E.W. and Wislocki, G.B.: Electronmicroscopic observations on the placenta
of the cat. J. Biophys. and Biochem. Cytol. 2:734-754, 1956.
Denker,
H.-W., Eng, L.A. and Hammer, C.E.: Studies on the early development and
implantation in the cat. II. Implantation: Proteinases. Anat. Embryol.
154:39-54, 1978.
Evermann,
J.F., Baumgartner, L., Ott, R.L., Davis, E.V. and McKeirnan, A.J.: Characterization
of a feline infectious peritonitis virus isolate. Vet. Pathol. 18:256-265,
1981.
Fox,
J.G., Cohen, B.J. and Loew, F.M.: Laboratory Animal Medicine. Academic
Press, Orlando, Florida, 1984.
Huxtable,
C.R., Duff, B.C., Bennett, A.M., Love, D.N. and Butcher, D.R.: Placental
lesions in habitually aborting cats. Vet. Pathol. 16:283-291, 1979.
Jones, B.R.: Feline infectious peritonitis: a review. New Zealand Vet.
J.: 23:221-225, 1975.
Garner,
F.M. and Lingeman, C.H.: Mast-cell neoplasms of the domestic cat. Path.
Vet. 7:517-530, 1970.
Gray,
A. P.: Mammalian Hybrids. A Check-list with Bibliography. 2nd ed. Commonwealth
Agricultural Bureaux, Farnham Royal, 1972.
Hare,
W.C.D., Weber, W.T., McFeely, R.A. and Yang, T.-J.: Cytogenetics in the
dog and cat. J. Small Anim. Pract. 7:575-592, 1966.
Hayes,
H.M. and Priester, W.A.: Feline infectious anaemia. Risk by age, sex and
breed; prior disease; seasonal occurrence; mortality. J. small Anim. Pract.
14:797-804, 1973.
Hsu,
T.C. and Rearden, H.H.: Further karyological studies on felidae. Chromosoma
16:365-371, 1965.
Huxtable,
C.R., Duff, B.C., Bennett, A.M., Love, D.N. and Butcher, D.R.: Placental
lesions in habitually aborting cats. Vet. Pathol. 16:283-291, 1979.
Johnstone,
S.D., Buoen, L.C., Madl, J.E., Weber, A.F. and Smith, F.O.: X-chromosome
monosomy (37,X0) in a Burmese cat with gonadal dysgenesis. J. Amer. Vet.
Med. Assoc. 182:986-989, 1983.
Jones,
B.R.: Feline infectious peritonitis: a review. New Zealand Vet. J.: 23:221-225,
1975.
Kehrer, A.: Zur Entwicklung und Ausbildung des Chorions der Placenta zonaria
bei Katze, Hund und Fuchs. Z. Anat. Entwickl.-Gesch. 143:25-42, 1973.
Leiser, R.: Blastocystenimplantation bei der Hauskatze. Licht- und elektronenmikroskopische
Untersuchung. Zbl. Vet. Med. C, Anat. Histol. Embryol. 8:79-96, 1979.
Ludwig,
K.S.: Zur vergleichenden Histologie des
Allantochorion. Rev. Suisse Zool. 75:819-831, 1968.
Malassiné,
A.: Evolution ultrastructurale du labyrinthe du placenta de chatte. Anat.
Embryol. 146:1-20, 1974.
Malassiné, A.: Étude ultrastructurale du paraplacenta de
chatte: mécanisme de l'érythrocytophagocytose par la cellule
chorionique. Anat. Embryol. 151:267-283, 1977.
Malouf, N., Benirschke, K. and Hoefnagel, D.: XX/XY chimerism in a tri-colored
male cat. Cytogenetics 6:228-241, 1967.
Miller,
W.J. and Hollander, W.F.: The sex-linked black cat fallacy: a textbook
case. J. Hered. 77:463-464, 1986.
Mossman,
H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Murphy,
W.J., Sun, S., Chen, Z.-C., Pecon-Slattery, J. and O'Brien, S.J.: Extensive
conservation of sex chromosome organization between cat and human revealed
by parallel radiation hybrid mapping. Genome Res. 9:1223-1230, 1999a.
Murphy,
W.J., Menotti-Raymond, M., Lyons, L.A., Thompson, M.A. and O'Brien, S.J.:
Development of a feline whole genome radiation hybrid panel and comparative
mapping of human chromosome 12 and 22 loci. Genomics 57:1-8, 1999b.
Newman,
A., Bush, M., Wildt, D.E., v. Dam, D., Frankenhuis, M.Th., Simmons, L.,
Phillips, L. and O'Brien, S.J.: Biochemical genetic variation in eight
endangered or threatened felid species. J. Mammal. 66:256-267, 1985.
Norby,
D.E. and Thuline, H.C.: Gene action in the X chromosome of the cat (Felis
catus L..). Cytogenetics 4:240-244, 1965.
Nowak,
R.M. and Paradiso, J.L.: Walker's Mammals of the World. 2 Vol. The Johns
Hopkins University Press, Baltimore, 1983.
O'Brien,
S.J.: The extent and character of biochemical genetic variation in the
domestic cat. J. Hered. 71:2-8, 1980.
O'Brien,
S.J., Haskins, M.E., Winkler, C.A., Nash, W.G. and Patterson, D.F.: Chromosomal
mapping of beta-globin and albino loci in the domestic cat. A conserved
mammalian chromosome group. J. Hered. 77:374-378, 1986.
O'Brien,
S.J., Wienberg, J. and Lyons, L.A.: Comparative lessons from cats. TIG
13:393-399, 1997.
Ramsey,
E.M.: The Placenta. Human and Animal. Praeger Publishers, NY, 1982.
Ramsey,
E. M.: The Placenta of Laboratory Animals and Man. Holt, Rinehart and
Winston, N.Y., 1975.
Reinert,
H. and Smith, G.K.A.: Establishment of an experimental cat breeding colony.
Nature 209:1005-1008, 1966.
Robinson,
R.: Genetics for Cat Breeders. 2nd. ed. Pergamon Press, NY 1971.
Roubin,
M., de Grouchy, J. and Klein, M.: Les félidés: Évolution
chromosomique. Ann. Génét. 16:233-245, 1973.
Sandström,
B., Westman, J. and Öckerman, P.A.: Glycogenosis of the central nervous
system in the cat. Acta Neuropath. (Berlin) 14:194-200, 1969.
Starck, D.: Lehrbuch der Speziellen Zoologie. Vol. II, Part 5/2. Gustav
Fischer Verlag, Jena, 1995.
Thenius,
E.: Zur Phylogenie der Feliden (Carnivora, Mamm.). Z. zool. Syst. Evolutionsforschung
5:129-143, 1967.
Tiedemann,
K.: The amniotic, allantoic and yolk sac epithelia of the cat: SEM and
TEM studies. Anat. & Embryol. 158:75-94, 1979.
Wimsatt, W.A.: Some aspects of the comparative anatomy of the mammalian
placenta. Amer. J. Obstet. Gynecol. 84:1568-1594, 1962.
Wislocki,
G.B.: Experimental studies on fetal absorption, II. Behaviour of the fetal
membranes and placenta of the cat toward colloidal dyes injected into
the maternal blood stream. Carnegie Contrib. to Embryol. 11: 1920
Wurster-Hill,
D.H. and Gray, C.W.: Giemsa banding patterns in the chromosomes of twelve
species of cats (Felidae). Cytogenet. Cell Genet. 12:377-397, 1973.
Wynn,
R.M. and Björkman, N.: Ultrastructure of the feline placental membrane.
Amer. J. Obstet. Gynecol. 102:34-43, 1968.
Zhemkova, Z.P.: The use of sex chromatin in identifying embryonic and
maternal tissues in the placenta: New observations on the haemochorial
nature of the cat placenta. J. Embryol. Exp. Med. 10:127-139, 1962.
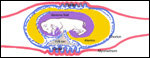
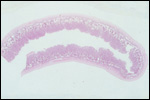
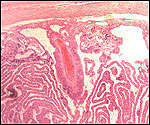
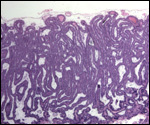
18)
Other findings of interest
|