| (Clicking
on the thumbnail images will launch a new window and a larger version
of the thumbnail.) |
| Last updated: July 10, 2004. |
Hydrochaeris hydrochaeris
by Peter Kaufmann
Order: Rodentia
Family: Caviidae
1) General Zoological Data
Capybaras ("carpinchos") are the largest rodents and perhaps derivatives of a much larger species still, Phoberomys, that is now extinct (Sanchez-Villagra et al., 2003). Their body weights normally range from 30 to 50 kg. Capybaras belong to the South American suborder of caviomorph rodents. They live in large groups in and along rivers and lakes in the lowlands of Panama, Colombia, Venezuela and Brazil. Family groups of around six animals are usual; they are commonly seen in the Pantanal of Brazil. The Panamanian subspecies is smaller. Often the genus is called Hydrochoerus as well. Its name is derived from the commonly applied reference as "water pig" (Gotch, 1979).
In the past decades, capybaras have raised the scientific interest of placentologists for a certain period, mainly because of the fact that they are members of the interesting suborder of caviomorph rodents. This suborder is characterized by an enormous diversity in body weight, ranging from the small degu (100 to 150 g) to the sheep-shaped capybara (mean weight is 40,000 g). Moreover, these rodents are charaterized by a rather long pregnancy period, by a hormonal control of pregnancy which is similar to that of the human and, in particular, by their striking homogeneity of placental structure, irrespective of the enormous range in weights - from <1 g (degu) to > 100 g (capybara); they are structurally more or less identical. The longevity of capybaras in captivity is up to 12 years (Nowak, 1999).
 |
Adult capybara. |
(a) In the sheep, which is a species that is easy to keep and breed and that is large enough for sophisticated in vivo experiments such as chronic catheterization of placental vessels for studies of placental transport. The main disadvantage of the sheep, however, is the fact that its placenta is cotyledonary in organization and epitheliochorial in its barrier structure and it thus shows only minimal resemblance to that of the human placenta. Therefore, transport data can be transferred to human placentation only with some reservation.
(b) In contrast to the sheep, there is then the small, handy, easy-to-breed guinea pig which is characterized by having a discoidal, hemochorial placenta with a maternal/fetal barrier largely identical to that of the human placenta. The small body size, however, makes short term experiments difficult, and chronic catheterization nearly impossible.
It is therefore easily understandable that the suggestion coming to placentologists about the existence of a sheep-sized "giant guinea pig" raised enormous expectations. A caviomorph rodent with 30 to 50 kg body size, 1,500 g neonatal weight, 150 days pregnancy length, placental vessels similar to water pipes, and all this together with a discoidal, hemochorial placenta would fulfill even the greatest expectations of every experimentator.
When we started our capybara colony in Aachen, our only fear was that reproduction of these animals might be the problem. This, we were told, was the case in most zoos. We started our colony with two males from a German zoo and three females were imported from Venezuela. In order to develop a strategy how to mate these allegedly difficult to breed animals, and in order to prevent damage to our costly females, males and females were kept in neighboring rooms in our animal facility for the first night. They were separated from each other by two 6 cm strong wooden doors because unexpectedly the perfect cooperation between Lufthansa and the zoo in Caracas, the females arrived earlier than the steel armored cages ordered. The next morning we found that both doors, including considerable parts of the animal laboratories, were ruined and all three females were pregnant.
Rapid reproduction, in fact, became a problem. Our breeding colony grew more quickly than expected and than we could afford, food-wise and space-wise. This was particulalrly true considering the delicate care for big, fierce tropical animals in the cold and rainy climate of Germany. Moreover the animal handlers had problems with our growing colony so that it was finally housed in a high-fenced garden with lakes. It quickly became an attraction for school classes and tourists. The real experimental problem, however, was caused by the fact that capybaras are extremely powerful, have not been truly domesticated, and they are furious animals which do not even fear the jaguar in their South-American habitat. Nor did they fear us. They can be enormously patient and playful so long as you pet them. But they turned into beasts and flattened an entire laboratory when we simply wanted to have a drop of blood.
A "laboratory animal" that cannot be immobilized without general anesthesia, that required general anesthesia for every simple test or treatment, that removed every implanted catheter within hours, even when implanted on its back, and that responded with abortion or preterm delivery to every second general anesthesia given certainly does not fulfill the expectations of a placentologist. Meanwhile our beautiful colony has become the pride of a small zoo to which we had donated it. In spite of this scientific disappointment, the few facts which we were able to collect are listed subsequently.
2) General Gestational Data
Length of gestation: 150 - 155 days
Litter size: 1 - 8, mean 5 (Weir, 1974), under laboratory conditions 3
Body weight (non pregnant) : >30 kg, at full term: up to 65 kg
Fetal weight at full term: 1,500 g
Weight of placenta and membranes at full term: about 100 g.
3) Implantation
Interstitial, antimesometrial implantation around day 6 post conception.
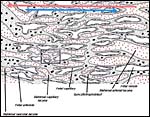
The general placental type is discoidal, labyrinthine, hemomonochorial, chorioallantoic main placenta with a specialized subplacenta and a separate yolk sac placenta.
The allantois is only used for the formation and vascularization of the chorioallantoic placenta (main placenta and subplacenta). Miglino et al. (2004) have presented a remarkable comparative study of the placentas of agouti, capybara, guninea pig, paca and rock cavy that gives superb details of the vascular organization of these organs.
The exchange area, the labyrinth, is identical to that of the guinea pig and other caviomorph rodents: it is the labyrinth that is composed of about 100 finger-like lobes. Each lobe measures from 3 to 6 mm in diameter and 15 to 20 mm in length.
The maternal blood flows from an arterial lacuna located in the lobar center centrifugally via a web of trophoblast-lined blood lacunae to the periphery of the lobe, where it is drained by a web of venous lacunae which pass the interlobar trophoblast.The blood flow arrangement represents a perfect counter-current exchange system.
The cord is composed of a vitelline and allantoic portion as shown in the first schematic. It is short and similar to other caviomorphs. (See chapters on guinea pig and nutria).
7) Uteroplacental Circulation
Each implantation site is supplied by several highly coiled uteroplacental
arteries with several millimeters of luminal diameter. This high degree
of dilatation of uteroplacental arteries reaches from the placenta back
close to the arcade artery which connects ovarian and uterine arteries.
Whether this enormous degree of arterial dilatation is due to endovascular
trophoblast invasion or to peritoneal trophoblast invasion remains to
be answered. For further details see the chapter on guinea pig.
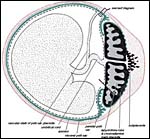
As in the guinea pig, the yolk sac placenta replaces the thin, non-placental, part of the blastocystic trophoblast vesicle. Together with the chorioallantoic placenta it forms the fetal membranes. Its endodermally-covered and fetally vascularized folds are in intimate contact with the endometrial surface epithelium. Those features are diagrammed next and details may be obtained from the publication by Miglino et al. (2002).
 |
Schematics of visceral yolk sac placenta (green) of caviomorph rodent attached to uterine wall (red), and amnion (gray). |
All caviomorph rodents are characterized by an unusual degree of trophoblast invasion. Trophoblast cells, uninucleated and multinucleated in type, do not only invade the endometrium and myometrium, but regularly penetrate the uterine wall and spread as a thin, monocellular layer across the peritoneal cavity. In the guinea pig, it has been shown that trophoblast at term replaces large parts of the peritoneal mesothelium (Nanaev et al., 1995). Our few observations in the capybara suggest the same highly invasive behavior to be present in this species. It remains unknown whether or not the endovascular trophoblast invasion along the uteroplacental arteries is as aggressive as it is in the guinea pig. It might be mentioned, however, that in nutria it is identical (see chapter on Nutria) and may be a feature of most if not all South American hystricomorph rodents.
10) Endometrium
The endometrial stromal cells decidualize where they come into contact
with trophoblast.
11) Various Features
As in all caviomorph rodents, a massive subplacenta can be found beneath
the chorioallantoic main placenta of the capybara (Miglino et al., 2002).
Neither we nor the latter authors found any differences when comparing
it with the subplacenta of the guinea pig (for details of the latter see
Kaufmann & Davidoff, 1977). In short, the capybara subplacenta is
composed of a complexly folded layer of syncytiotrophoblast with a complete
layer of cytotrophoblast beneath; some connective tissue cells which embed
scarce fetal capillaries underneath the cytotrophoblast, and a web-like
system of maternal lacunae penetrate the syncytiotrophoblast.
Maternal blood flow in these lacunae ceases already a few weeks post conception.
Fetal capillarization of the subplacenta starts only thereafter. Consequently,
the subplacenta is in no stage of pregnancy simultaneously supplied by
both, maternal and fetal blood flow. Therefore, it cannot serve as an
exchange organ. It degenerates a few days prior to parturition and leaves
a mass of cellular debris at the site of placental separation. Its functional
relevance is still a mystery. Structural data suggest that it serves mainly
a secretory function. It seems to act as source for trophoblast invasion.
12) Endocrinology
We presume this to be similar to that of the guinea pig.
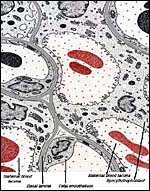
Capybaras have 66 chromosomes and, like other South American rodents, their X-chromosome is unusually large (+/- 10% of genome) (Hsu & Benirschke, 1974; Wurster et al., 1971). A molecular study of pancreatic ribonuclease genes has shown a duplication in guinea pigs but only a single copy is found in capybara and cuis (Beintema & Neuteboom, 1983). Hybrids have not been reported.
 |
Karyotypes of male and female cpybaras. |
We know of no relevant studies.
15) Pathological Features
Griner (1983) found myocardial infarcts, nephritis, gastric ulcers and
a variety of infectious as causes of death. Numerous ectoparasites and
hematological parasites have been reported. Access to these publications
is available through PubMed. In none of Griner's animals was a scrotum
present; the testes were either intraabdominal or in the inguinal canal.
Sex determination is best done by everting the penis. Scurvy may be induced
by vitamin C deficiency (Cueto et al., 2000).
16) Physiological Data
Paula et al. (1999) studied the seminiferous epithelium of capybaras,
including the meiotic time. The length of the spermatogenic cycle was
measured to be 53.6 days. Vitamin C dependence was determined by Cueto
et al. (2000). Remarkably, females have 14 teats (Hayssen et al.,1993).
Others have reported 10 nipples.
17)
Other Resources
Fibroblast cell strains are available from CRES
at the San Diego Zoo by contacting Dr. Oliver Ryder at oryder@ucsd.edu.
18) Other Remarks - What additional
information is needed?
More detailed studies are needed to secure the presumption that the extrauterine
trophoblast proliferation is similar to that of nutria and guinea pig.
Collection of early implantation stages and endocrine data is essential.
Acknowledgement
Prof. Werner Kuepper, Animal Devices, Medical Faculty, University of Technology
Aachen is gratefully acknowledged for taking care of our capybara breeding
colony.
References
Beintema, J.J. and Neuteboom, B.: Origin of the duplicated gene in guinea-pig:
comparison of the amino acid sequences with those of two close relatives:
capybara and cuis ribonuclease. J. Mol. Evol. 19:145-152, 1983.
Cueto, G.R., Allekotte, R. And Kravetz, F.O.: Scurvy in capybaras in captivity in Argentine. J. Wild. Dis. 36:97-101, 2000.
Gotch, A.F.: Mammals - Their Latin Names Explained. Blandford Press, Poole, Dorset, 1979.
Griner, L.A.: Pathology of Zoo Animals. Zoological Society of San Diego, San Diego, California, 1983.
Hayssen, V., van Tienhoven, A. and van Tienhoven, A.: Asdell's Patterns of Mammalian Reproduction: a Compendium of Species-specific Data. Comstock/Cornell University Press, Ithaca, 1993.
Hsu, T.C. and Benirschke, K.: An Atlas of Mammalian Chromosomes. Springer-Verlag, New York. Vol. 8, Folio 378, 1974.
Kaufmann, P. and Davidoff, M.: The guinea-pig placenta. Adv. Anat. Embryol. Cell Biol. 53: 1-91, 1977.
Lopez Barbella, S.: Determination of the estrous cycle of the capybara (Hydrochoerus hydrochaeris). [In Spanish]. Acta Cient. Venz. 33:497-501, 1982 (not available to us).
Miglino, M.A., Carter, A.M., dos Santos,
R.H. and F. Machado, M.R.: Placentation in the capybara (Hydrochaerus
hydrochaerus), agouti (Dasyprocta aguti) and paca (Agouti
paca). Placenta 23:416-428, 2002.
Miglino, M.A., Carter, A.M., Ambrosio, C.E., Bonatelli, M., de Oliveira, M.F., dos Santos, Ferraz, R.H., Rodrigues, R.F. and Santos, T.C.: Vascular organization of the hystricomorph placenta: a comparative study in the agouti, capybara, guinea pig, paca and rock cavy. Placenta 25:438-448, 2004.
Nowak, R.M.: Walker's Mammals of the World. 6th ed. The Johns Hopkins Press, Baltimore, 1999.
Paula, T.A., Chiarini-Garcia, H. And Franca, L.R.: Seminiferous epithelium cycle and its duration in capybaras (Hydrochoerus hydrochaeris). Tissue Cell. 31:327-334, 1999.
Sanchez-Villagra, M.R., Aguilera, O. and Horovitz, I.: The anatomy of the world's largest extinct rodent. Science 301:1708-1710, 2003.
Weir, B.J.: Reproductive characteristics of hystricomorph rodents. Symp. Zool. Soc. London 34:265-301, 1974.
Wurster, D.H., Snapper, J.R. and Benirschke, K.: Unusually large sex chromosomes: new methods of measuring and description of karyotypes of six rodents (Myomorpha and Hystricomorpha) and one Lagomorpha (Ochotonidae). Cytogenetics 10:153-176, 1971.