| (Clicking
on the thumbnail images will launch a new window and a larger version
of the thumbnail.) |
| Last updated: July 4, 2004. |
Cebus albifrons albifrons
Order: Primates
Family: Cebidae
1) General Zoological Data
Six species are recognized according to Nowak (1999), all from Central or South America and these are further subdivided by Groves (2001). In general, their physiological and pathological parameters appear to be very similar. The evolutionary split of catarrhines and platyrrhines (25-35 MYA) was inferred by Courseaux et al. (2003) from the duplicated euchromatic segments. Many experimental colonies are kept in Primate Research Centers and by private individuals. They are easily trained animals that are sometimes also used to support handicapped persons. Apparent infanticide has been reported by Manson et al. (2004). Adults weigh between 1,000 and 4,000 g and possess a moderately useful prehensile tail (Lemelin, 1995).
 |
White-fronted capuchin monkey at San Diego Zoo. |
 |
Mother and infants whose placenta is here described. |
2) General Gestational Data
Gestation lasts 160-180 days and ends with the birth of a singleton weighing 200-250 g. Twinning has been reported in 2.4% of captive colonies of C. apella by Leighty et al. (2004). 45% of these twins will die neonatally, as compared to 16% of singletons but subsequently they do well. Longevity is up to 45-55 years. Neonatal growth and other reproductive phenomena have been examined by Fragaszy & Adams-Curtis (1998). Sperm migration through the uterus was approximately one hour (Ortiz et al., 1995).
3) Implantation
This placenta is said to have an antimesometrial implantation but one lobe locates anteriorly, the other on the posterior wall of the unicornuate uterus. Membranous vessels connect the two lobes.
4) General Characterization of the Placenta
I have had one placenta available for study; it weighed 63 g. This was a two-lobed, discoid, hemochorial placenta with long, slender villi, resembling those of spider monkeys. The villi are also referred to as being trabecular. In contrast to the callithricid monkeys, no hematopoietic foci were found in this placenta. The cord inserted marginally on the major lobe and vessels traversed in the membranes to the other lobe. Its appearance is like that described by Soma (1978) and Young (1972). This placenta was meconium-stained, although the neonate survived.
 |
Capuchin placenta, courtesy H. Soma, Tokyo . |
 |
Complete cross section of one lobe of capuchin placenta with fetal surface above. |
5) Details of fetal/maternal barrier
The surface of the mature villi is covered with syncytiotrophoblast that is bathed in maternal intervillous blood. In the term placenta there is no cytotrophoblast visible although fine-structurally it must be present. The villi contain few macrophages, the “Hofbauer” cells, and are otherwise made of delicate connective tissue.
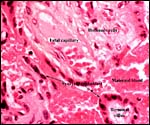 |
Capuchin placental relationship to intervillous circulation. |
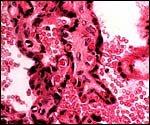 |
Mature villi surrounded by maternal intervillous blood. |
6) Umbilical cord
The capuchin umbilical cord has variable insertion sites. It contains two arteries and two veins, in addition to a small allantoic duct, but smaller vessels as in ungulates are absent. Young 1972) described fusion of the two veins in the middle of the cord in one case. Spatz (1968) found the cord to be 10 cm long. The small ducts, mostly the allantoic duct, may be present but that is apparently very variable. The vitelline duct is usually diminutive.
7) Uteroplacental circulation
No studies are known to me, except that Ramsey (1982) stated that Cebidae do not possess spiral arterioles in the endometrium.
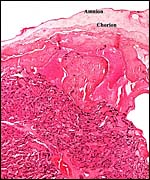 |
This is from the margin of the capuchin placenta with the degenerating (due to meconium) amnion above. The membranes extend to the right but have no atrophic villi. |
8) Extraplacental membranes
The amnion is avascular and it is pressed against the underlying chorion. The amnion is avascular, the chorion carries the large fetal vessels. The amnionic epithelium is single-layered and squamous. Foci of squamous metaplasia may be present. Decidua capsularis was not seen in this placenta and there were no atrophic villi as are seen in the membranes of human placentas. The outer surface of the chorion is covered by a thin layer of cytotrophoblast, so-called “X-cells”. There was no allantoic sac in this placenta but Young (1972) reported its presence in 5 of 6 C. apella placentas. Persistent yolk sacs are uncommon, as are remnants of their ducts.
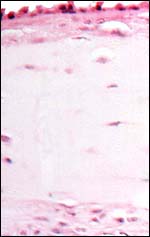 |
The edematous nature of the membrane and the degenerating amnionic epithelium are due to meconium toxicity. |
9) Trophoblast external to barrier
I have not had an implanted placenta available for study. Thus, whether trophoblast invades the deciduas basalis is not known to me; however, Mossman (1987) stated it to be absent. In comparing this placenta with other cebid placentas, the basal infiltration would also have to be a shallow one. In this delivered placenta there was no decidua shed; the “floor” consisted of extravillous trophoblast (“X-cells”) only. It was accompanied by some syncytial cells. Ramsey (1982) stated that there is a pronounced “epithelial plaque” at the periphery of cebid placentas; my specimen had merely extravillous trophoblast. This could conceivably be mistaken as epithelium.
10) Endometrium
The decidual transformation is apparently minimal and spiral arterioles are said to be absent (Ramsey, 1982).
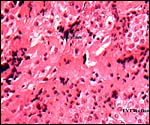 |
Placental floor of delivered placenta; it consists mostly of extravillous trophoblast with little fibrinoid and no decidua. |
11) Various features
There are no specific unusual features in capuchin placentas.
12) Endocrinology
The placenta produces chorionic gonadotropin. Postnatal testosterone and the production of other androgens was studied by Rey et al. (1995). An interesting study of adrenal cortisol production was undertaken by Torres-Farfan et al. (2004). They found that fetuses whose mothers are exposed to constant light (and thus induced melatonin suppression) had no effect on fetal DHAS production (as inferred from maternal estradiol levels). Newborns, however, of these mothers had twice as much plasma cortisol. Thus, melatonin (and its regulation by light) affects fetal and neonatal cortisol levels.
The menstrual cycle is 18-22 days long and implantation occurs on day 5 after ovulation (Jones et al., 2001). These authors studied the endometria and found glands deep in the myometrium, and Muc1 expression on epithelial surfaces. A variety of endocrine studies were done on small quantities of urine; thus by Nagle et al. (1979; 1980; 1983; 1989), Lasley et al. (1980), Czekala et al. (1981), Hodges et al. (1979; 1981) and Castellanos & McCombs (1968).
13) Genetics
Capuchin monkeys have usually had 54 chromosomes and they have been studied extensively by de Boer (1974) who provided the chromosomes shown below. The one exception (2n=52 in a C. capucinus ) was studied by Egozcue et al. (1967 – cited by de Boer), but it lacks confirmation to date. Numerous hybrids between the various species were recorded by Gray (1972). Garcia et al. (1999) studied the “constitutive heterochromatin” (C-bands) of capuchins and chimpanzees and compared these with human chromosomes. They found widely scattered amounts of centromeric heterochromatin as opposed to little variation of the noncentromeric regions. In a later study (2002), these authors compared capuchins and spider monkeys with ZOO-FISH. Courseaux et al. (2003) studied segmental chromosome duplications of human #5 and drew evolutionary conclusions. Chiu et al. (1999) described the recruitment of gamma-globin genes in capuchins and marmosets.
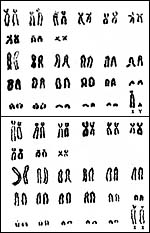 |
Karyotypes of male and female Cebus albifrons (from Hsu & Benirschke, 1975). |
14) Immunology
The immunological response to experimental infections has been recorded and is detailed under the next section. Ferrari et al. (2003) found that, while herpes virus (Epstein-Barr) immortalizes T-cells of tamarins, it did not do so in capuchin T-cells.
15) Pathological features
Two capuchins were reported as having oral squamous cell carcinomas (Grana et al., 1992). Renal lesions (glomerulonephritis and pyelonephritis) caused the death of 5 capuchins in Argentina (Borda et al., 2000). A variety of parasites have been identified in capuchins, thus mites from the auditory canal (Guerim et al., 2001) and intestinal nematodes (Durette-Desset et al., 2001). These animals have also been used for experimental studies of Chagas disease with its myocardiopathy (Samudio et al., 1999). The immunological response to the infection was followed precisely in that study. Similarly, leishmaniasis and the immunological response to that experimental infection were topics of study by Garcez et al., (1997; 2001). Griner (1983) found a capuchin to have died from a myocardial infarct. Occasional parasitic infections, respiratory and renal causes of death are also listed.
16) Physiologic data
There is much information on the social aspects, handedness, CNS development and CNS function, and also on various anatomical aspects of capuchin monkeys. It is suggested that the reader look in PubMed for appropriate listings. The hematology and blood chemistry values of C. apella were provided by Riviello & Wirz (2001).
17) Other resources
Some cell strains of capuchin monkeys are available from CRES by contacting Dr. Oliver Ryder at oryder@ucsd.edu .
18) Other remarks – What additional Information is needed?
Early implantation stages have not been recorded to my knowledge.
Acknowledgement
The animal photographs in this chapter come from the Zoological Society of San Diego. I am grateful to Dr. Hiroaki Soma for allowing me to show the macroscopic picture of the placenta he published in 1978.
References
Borda, J.T., Nunes-Bastos, V., Perez-Escala, S. and Sanchez-Negrette, M.: Histopathological characterization of nephritides in the brown capuchin monkey, Cebus apella (Primates: Cebidae). Rev. Biol. Trop. 48:255-260, 2000).
Castellanos, H. and McCombs, H.L.: The reproductive cycle of the New World monkey: Gynecologic problems in a breeding colony. Fertil. Steril. 19:213-227, 1968.
Chiu, C.H., Gregoire, L., Gumucio, D.L., Muniz, J.A., Lancaster , W.D. and Goodman, M.: Model for the fetal recruitment of simian gamma-globin genes based on findings from two New World monkeys Cebus apella and Callithrix jacchus (Platyrrhini, Primates). J. Exp. Zool. 285:27-40, 1999.
Courseaux, A., Richard, F., Grosgeorge, J., Ortola, C., Viale, A., Turc-Carel, C., Dutrillaux, B., Gaudray, P. and Nahon, J.L.: Segmental duplications in euchromatic regions of human chromosome 5: a source of evolutionary instability and transcriptional innovation. Genome Res. 13:369-381, 2003.
Czekala , N.M. , Hodges, J.K. and Lasley, B.L.: Pregnancy monitoring in diverse primate species by estrogen and bioactive luteinizing hormone determination in small volumes of urine. J. Med. Primatol. 10:1-16, 1981.
De Boer, L.E.M.: Cytotaxonomic Studies in the Primate Suborders Prosimii and Platyrrhini. Bronder-Offset, Rotterdam , 1974.
Durette-Desset, M.C., Fribourg Blanc, L.A. and Vuong, P.N.: Molineus torulosus (Nematoda, Trichostringylina, Molineoidea) a parasite of neotropical primates: new morphological and histological data. Parasite 8:53-60, 2001.
Ferrai, M.G., Stevceva, L., Markham , P. and Franchini, G.: Species-specific transformation of T-cells by HV(MNE). Virology 317:299-307. 2003.
Fragaszy, D.M. and Adams-Curtis, L.E.: Growth and reproduction in captive tufted capuchins ( Cebus apella ). Amer. J. Primatol. 44:197-203, 1998.
Garcez, L.M., Silveira, F.T., el Harith, A., Lainson, R. and Shaw, J.J.: Experimental cutaneous leishmaniasis. IV. The humoral response of Cebus apella (Primates: Cebidae) to infections of Leishmania (Leishmania) amazonensis , L. (Viannia) lainsoni and L. (V.) braziliensis using the direct agglutination reaction. Acta Trop. 68:65-76, 1997.
Garcez, L.M., Goto, H., Ramos, P.K., Brigido, Mdo, C., Gomes, P.A., Souza, R.A., de Luca, P.M., Mendonca , S.C. , Muniz, J.A. and Shaw, J.J.: Leishmania (Leishmania) amazonensis -induced cutaneous leishmaniasis in the primate Cebus apella : a model for vaccine trials. Int. J. Parasitol. 32:1755-1764, 2002.
Garcia, F., Nogues, C., Garcia, M., Egozcue, J. and Ponsa, M.: Characterization of constitutive heterochromatin in Cebus apella (Cebidae, Primates) and Pan troglodytes (Hominidae, Primates): comparison to human chromosomes. Amer. J. Primatol. 49:205-221, 1999.
Garcia, F., Ruiz-Herrera, A., Egozcue, J., Ponsa, M. and Garcia, M.: Chromosomal homologies between Cebus and Ateles (primates) based on ZOO-FISH and G-banding comparisons. Amer. J. Primatol. 57:177-188, 2002.
Grana, D., Mareso, E. and Gomez, E.: Oral squamous cell carcinoma in capuchin monkeys ( Cebus apella ) Report of two cases. J. Med. Primatol. 21:384-386, 1992.
Gray, A.P.: Mammalian Hybrids. A Check-list with Bibliography. 2 nd edition. Commonwealth Agricultural Bureaux Farnham Royal, Slough , England , 1972.
Griner, L.A. : Pathology of Zoo Animals. Zoological Society of San Diego , San Diego , California , 1983.
Groves , C.: Primate Taxonomy. Smithsonian Institution, Washington , DC , 2001.
Guerim, L., Gazeta G.S., Serra-Freie , N.M. , de Sa, L.M. and Cato Dias, J.L.: Cebus apella (Primata: Cebidae) a new host for Fonsecalges johnjadini (Acari: Psoroptidae, Cebalginae) with a description of anatomopathological aspects. Mem. Inst. Oswaldo Cruz 96:479-481, 2001.
Hodges, J.K., Czekala , N.M. and Lasley, B.L.: Estrogen and luteinizing hormone secretion in diverse primate species from simplified urine analysis. J. Med. Primatol. 8:349-364, 1979.
Hodges, J.K., Gulick, B.A., Czekala , N.M. and Lasley, B.L.: Comparison of urinary oestrogen excretion in South American primates. J. Reprod. Fertil. 61:83-90, 1981.
Hsu, T.C. and Benirschke, K.: An Atlas of Mammalian Chromosomes. Vol. 9; Folio 447, 1975. Springer-Verlag , N.Y.
Jones, C.J., Ortiz , M.E. , Croxatto, H.B., Manzur, A., Slevin, G. and Aplin, J.D.: Muc1 and glycan expression in the oviduct and endometrium of a New World monkey, Cebus apella . Biol. Reprod. 64:1535-1544, 2001.
Lasley, B.L., Hodges, J.K. and Czekala , N.M. : Monitoring the female reproductive cycle of great apes and other primate species by determination of oestrogen and LH in small volumes of urine. J. Reprod. Fertil. Suppl. 28:121-129, 1980.
Leighty, K.A., Byrne, G., Fragaszy, D.M., Visalberghi, E., Welker, C. and Lussier, I. : Twinning in tufted capuchins ( Cebus apella ): rate, survivorship, and weight gain. Folia Primatol. 75:14-18, 2004.
Lemelin, P.: Comparative and functional myology of the prehensile tail in New World monkeys. J. Morphol. 224:351-368, 1995.
Manson, J.H., Gros-Louis, J. and Perry, S.: Three apparent cases of infanticide by males in wild white-faced capuchins ( Cebus capucinus ). Folia Primatol. 75:104-106, 2004.
Mossman, H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Nagle , C.A. and Denari, J.H.: The Cebus Monkey ( Cebus apella ). Reproduction in New World Primates, pp. 39-67. J. Hearn ed., MTP Press, Lancaster, England, 1983.
Nagle , C.A. and Denari, J.H. Quiroga, S., Riarte, A., Merlo , A., Germino, N.I., Gomez-Argana, F. and Rosner, J.M. : The plasma pattern of ovarian steroids during the menstrual cycle in capuchin monkeys ( Cebus apella ) Biol. Reprod. 21:979-983, 1979.
Nagle , C.A. , Riarte, A., Quiroga, S., Azorero, R.M., Carril, M., Denari, J.H. and Rosner, J.M . : Temporal relationship between follicular development, ovulation, and ovarian hormone profile in the capuchin monkey ( Cebus apella ). Biol. Reprod. 23:629-635, 1980.
Nagle ,, C.A. , Paul, N., Mazzoni, I. , Quiroga, S., Torres, M., Mendizabal, A.F. and Farinati, Z.: Interovarian relationship in the secretion of progesterone during the luteal phase of the capuchin monkey ( Cebus apella ). J. Reprod. Fertil. 85:389-396, 1989.
Nowak, R.M.: Walker 's Mammals of the World. 6 th ed. The Johns Hopkins Press, Baltimore, 1999.
Ortiz , M.E. , Gajardo, G., Leon , C.G., Herrera, E., Valdez , E.: Croxatto, H.B.: Sperm migration through the female genital tract of the New World monkey Cebus apella . Biol. Reprod. 52:1121-1128, 1995.
Ramsey, E.M.: The Placenta. Human and Animal. Praeger Publishers, New York , 1982.
Rey, R., Campo, S., Ayuso, S., Nagle, C. and Chemes, H.: Testicular steroidogenesis in the Cebus monkey throughout postnatal development. Biol. Reprod. 52:997-1002, 1995.
Riviello, M.C. and Wirz, A.: Haematology and blood chemistry of Cebus apella in relation to sex and age. J. Med. Primatol. 30:308-312, 2001.
Samudio, M., Montenegro-James, S., Kasamatsu, E., Cabral, M., Schinini, A., Rojas de Arias, A. and James, M.A.: Parasite Immunol. 21:451-460, 1999.
Soma, H.: Comparative Placentology. In, Modern Obstetrics and Gynecology. Pp. 123-159. Nakayama Publ. Tokyo (in Japanese), 1978.
Spatz, W.B.: Nabelschnur-Längen bei Insektivoren und Primaten. Z. Säugetierk. 33:226-239, 1968.
Torres-Farfan, C., Richter, H.G., Germain, A.M., Valenzuela, G.J., Campino, C., Rojas-Garcia, P., Forcelledo, M.L., Torrealba, F. and Seron-Ferre, M.: Maternal melatonin selectively inhibits cortisol production in the primate fetal adrenal gland. J. Physiol. 554(Pt.3):841-856, 2004.
Young, A.: The primate umbilical cord with special reference to the transverse communicating artery. J. Human Evol. 1:345-359, 1972.
