| |
Two
hybrids with the sitatunga (Tragelaphus spekei) have been described
from the Antwerp zoo (Gray, 1972). Their gestation lasted 309 days and Cesarean
section was necessary for delivery. One female hybrid produced a female
offspring when mated with a male sitatunga.
14) Immunology
I am not aware of any immunological study in Tragelaphinae.
15) Pathological
features
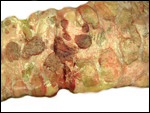
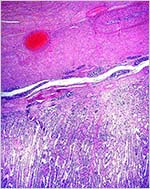
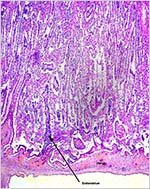
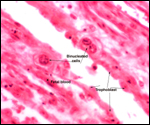
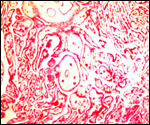
One common illness in hoofed species, including the Tragelaphinae, is
the occurrence of "white muscle disease", an acute skeletal
myopathy that occurs especially after strenuous exercise and capture (hence
it is often called "capture myopathy"). This has been aptly
summarized by Heldstab & Ruedi (1980).
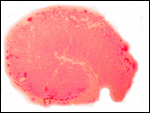
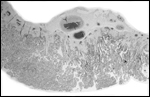
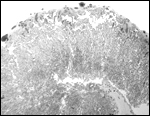
A congenital defect of diaphragmatic hernia was identified in San Diego
(see Benirschke et al., 1982). This neonate possessed one each of the
two X-chromosome morphotypes. It is unlikely, however, that the different
X-chromosome types were related to the anomaly.
Tragelaphinae are apparently susceptible to develop a scrapie-like encephalopathy.
This was reported twice in greater kudus by Kirkwood et al. (1992, 1994).
In one case the disease may have been transmitted in utero.
16) Physiologic
data
There are no such studies on bongos. Methods of immobilization were discussed
by Haigh (1976), and a Cesarean section has been described by Bush et
al. (1973). Pospisil et al. (1984) reported hematological values on these
species. They were generally similar to those of humans.
17) Other
resources
Cell strains from many specimens of this species and related tragelaphines
are available from CRES
at the Zoological Society of San Diego.
18) Other
remarks - What additional Information is needed?
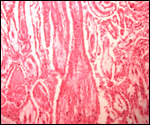
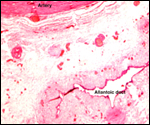
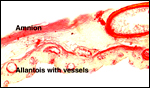
More placentas need to be studied to better understand the length of the
umbilical cord, weights, and possible diseases. It is especially important
to obtain more rapidly fixed material than was possible for me to obtain.
Acknowledgement
Most of the animal photographs in these chapters come from the Zoological
Society of San Diego. I appreciate also very much the help of the pathologists
at the San Diego Zoo.
References
Benirschke, K., Kumamoto, A.T., Esra, G.N. and Crocker, K.B.: The chromosomes
of the bongo, Taurotragus (Boocerus) eurycerus. Cytogenet.
Cell Genet. 34:10-18, 1982.
Bent, N.
and Reason, R.: A preliminary study of sex ratios in captive=-born ruminants.
Int. Zoo Yb. 36:223-228, 1998.
Bush, M.,
Montali, R.J., Gray, C.W. and Neeley, L.M.: Cesarean section in a Bongo
antelope. J. Amer. Vet. Med. Assoc. 163:552-553, 1973.
Doi, S.,
Shifrin, S., Santisteban, P., Montali, R.J., Schiller, C., Bush, M. and
Grollman, E.F.: Familial goiter in bongo antelope (Tragelaphus eurycerus).
Endocrinology 127:857-864, 1990.
Dresser,
B.L.: Embryo transfer in exotic bovids. Intern. Zoo Yearbook 24-25:138-142,
1986.
Gotch, A.F.: Mammals - Their Latin Names Explained. Blandford Press, Poole,
Dorset, 1979.
Gray, A.P.:
Mammalian Hybrids. A Check-list with Bibliography. 2nd edition. Commonwealth
Agricultural Bureaux Farnham Royal, Slough, England, 1972.
Haigh, J.C.:
The immobilization of bongo (Boocerus eurycerus) and other African
antelopes in captivity. Vet. Rec. 98:237-239, 1976.
Heldstab,
A. and Ruedi, D.: The occurrence of myodystrophy in zoo animals at the
Basle Zoological Garden. Pp. 27-34, In, The Comparative Pathology of Zoo
Animals, R.J. Montali and G. Migaki, eds., Smithsonian Institution Press,
Washington, D.C., 1980.
Hradecky,
P., Benirschke, K. and Stott, G.G.: Implications of the placental structure
compatibility for interspecies embryo transfer. Theriogenology 28:737-746,
1987.
Hradecky,
P., Mossman, H.W. and Stott, G.G.: Comparative histology of antelope placentomes.
Theriogenology 29:693-714, 1988.
Hradecky,
P., Mossman, H.W. and Stott, G.G.: Comparative development of ruminant
placentomes. Theriogenology 29:715-729, 1988.
Kirkwood,
J.K., Wells, G.A., Cunningham, A.A., Jackson, S.I., Scott, A.C., Dawson,
M. and Wilesmith, J.W.: Scrapie-like encephalopathy in a greater kudu
(Tragelaphus strepsiceros), which had not been fed ruminant-derived
protein. Vet. Rec. 130:365-367, 1992.
Kirkwood,
J.K., Cunningham, A.A., Austin, A.R., Wells, G.A. and Sainsbury, A.W.:
Spongiform encephalopathy in a greater kudu (Tragelaphus strepsiceros)
introduced into an affected group. Vet. Rec. 134:167-168, 1994.
Matthee,
C.A. and Robinson, T.J.: Cytochrome b phylogeny of the family Bovidae:
resolution within the alcelaphini, antilopini, neotragini, and tragelaphini.
Mol. Evol. 12:31-46, 1999.
Nowak, R.M.:
Walker's Mammals of the World. 6th ed. The Johns Hopkins Press, Baltimore,
1999.
Petit, P.,
Vermeesch, J.R., Marynen, P. and de Meurichy, W.: Comparative cytogenetic
study in the subfamily Tragelaphinae. Proc. 11th Europ. Coll. Cytogenet.
Domest. Anim. Pp. 109-113, 1994/5.
Pospisil,
J., Kase, F., Vahala, J. and Mouchova, I.: Basic haematological values
in antelopes - II. The hippotraginae and the tragelaphinae. Comp. Biochem.
Physiol. A 78:799-807, 1984.
Ralls, K.:
Tragelaphus eurycerus. In, Mammalian Species, No. 111, 1-4, 1978.
Amer. Soc. Mammal.
Schiller,
C.A., Montali, R.J., Doi, S. and Grollman, E.F.: Clinical and morphologic
findings of familial goiter in bongo antelope (Tragelaphus eurycerus).
Vet. Pathol. 32:242-249, 1995.
Thenius,
E.: Stammesgeschichte der Säugetiere (einschliesslich der Hominiden).
In Handbuch der Zoologie, J.G. Helmcke, D. Starck and H. Wermuth, eds.
Vol. 8/2:369-722, 1969, Walter de Gruyter & Co., Berlin.
Wallace,
C.: Chromosomal evolution in the antelope tribe Tragelaphini. Genetica
48:75-80, 1978.
Wurster,
D.H.: Sex-chromosome translocations and karyotypes in bovid tribes. Cytogenetics
11:197-207, 1972.
Xanten, W.A.
Jr.: Gestation period in the bongo (Boocerus eurycerus). J. Mammal.
53:232, 1972. |