| Last updated: August 30, 2010. |
Diceros bicornis
Order: Perissodactyla
Family: Rhinocerotidae
1) General Zoological Data
Rhinoceroses date back to the Eocene and were represented in America by several ancestral species. Asian and African precursors split about 26 Million years ago; American species died out. The black rhinoceros is perhaps the most severely endangered species of the five rhinocerotidae. It is estimated that some 3,610 animals survive but the decline between 1970 and 1992 is 96% (from 65,000 to 2,300). It is heavily poached but conservation efforts have increased their number (www.rhinos-irf.org). It distributes over South end central Africa . Its prehensile lips are perhaps the best-known feature of this browsing species. Black rhinos weigh from about 1,600 kg to 3,000 kg. Many zoological gardens have this species, have bred the animals and learned much about their pathophysiology to enhance survival. In a study of mitochondrial genes, Tougard et al. (2001) estimated that African and Asian rhino split some 26 Million years ago (see also Merenlender et al., 1989). Morales & Melnick (1994) used high resolution restriction site mapping of the mtDNA ribosomal region to examine the different and controversial classification of rhinos and concluded that the current nomenclature is adequate and useful. Diceros bicornis stands for two-horned, first in Greek and then in Latin (Gotch, 1979).
 |
Black rhinoceros with young at San Diego Wild Animal Park. |
2) General Gestational Data
One young is born only whose weight is 30-35 kg (Silberman & Fulton, 1979). The length of gestation is between 431 and 540 days, according to references provided by Hayssen et al. (1993).
3) Implantation
Early stages of implantation have not been studied. The diagram below is representative of all rhinocerotid placentations.
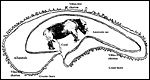 |
Diagram of rhinoceros membrane relationships. |
4) General Characterization of the Placenta
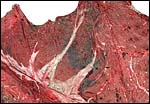
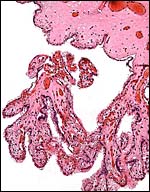
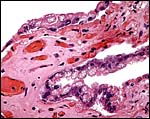
The placenta of this species of rhinoceros is very similar to that of the other rhinoceros species studied (see chapters on Indian and White rhinoceros). It is a diffuse, very thin sac that is studded on the outside with small villi. This neonate's placenta weighed 3,850 g, was 300 cm in greatest diameter and 0.2 cm in thickness. The cord inserted in the center and had three large vessels.
The rhinoceros placenta implants in both uterine horns, the fetus being located mostly in one horn, and the placenta then extends into the other horn. These are diffuse placentas without cotyledons, and with an epitheliochorial barrier character. A complete review of all publications on three species of rhinocerotidae placentas was published by Benirschke & Lowenstine (1995). Because of its inaccessibility, it is appended to the chapter on Indian rhinoceros.
5) Details of fetal/maternal barrier
This is a typical diffuse epithelial-chorial (appositional) placenta without evidence of invasion of the endometrium.
 |
|
 |
|
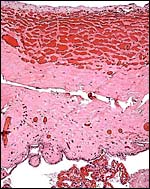 |
|
6) Umbilical cord
The umbilical cord measured 4 cm in length, had no twists and contained 3 large vessels and an allantoic duct. Its surface had no nodules. Since then additional placentas have become available. One measured 23 cm, the other 11 cm. in length.
7) Uteroplacental circulation
No descriptions are available.
8) Extraplacental membranes
Hippomanes did not accompany these membranes, nor were there the round, squamous patches on the amnion as present in white rhinoceros. The allantoic sac is large.
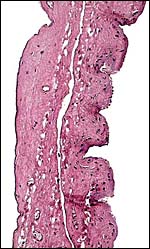 |
|
9) Trophoblast external to barrier
There is no trophoblast beyond the villous structures.
10) Endometrium
Decidualization has not been described.
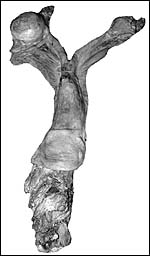 |
Reproductive tract of black rhinoceros (Courtesy, R. Montali). |
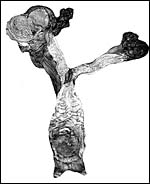 |
Reproductive tract of black rhinoceros (Courtesy, R. Montali). |
11) Various features
No special features are known to me.
12) Endocrinology
Pregnancy can be reliably established by measuring fecal progestational steroids (Schwarzenberger et al., 1993; Garniera et al., 1998), but not by vaginal cytology (Kock et al., 1991). The preservation of fecal samples for steroid study has been discussed many times and was controversial when samples had been imported for study. Now, Galama et al. (2004) have published a study that indicates reliable preservation of steroids when stored in methanol or after drying in a solar box cooker. The study was done in black rhinoceros, but should be equally applicable to other species. Reproductive parameters in zoological gardens have been delineated for the black rhinoceros by Carlstead et al. (1999). A workshop on white rhinoceros steroid determinations and induced ovulation was held in San Diego (Patton et al., 1998) and is available from that institution. Another comprehensive conference volume on rhinoceros biology is available from a 1991 conference (Ryder, 1993). Cycles and seasonality in black rhino reproduction was studied in Zimbabwe by fecal progesterone levels (Garnier et al., 2002). Lance et al. (2001) studied the types of steroid metabolites in feces of a pregnant black rhino but could not identify progesterone. By transrectal sonography, the ovarian events and gestational parameters were correlated to fecal progesterone by Radcliffe et al. (2001). These investigators also determined fetal sex sonographically and established parameters for aging fetal development from eye and foot measurements.
Stress can be assessed by the study of corticoids from fecal samples (Turner et al., 2002). Cycles can be ascertained by urinary steroid determinations, as shown by Hindle et al. (1992).
13) Genetics
A comprehensive review of ours in 1995 is difficult to access for most readers. For that reason it is scanned and appended to the very end of this chapter (Houck et al., 1995). The chromosome number of the black rhinoceroses is 2n=84 (Hungerford et al., 1967; Trifonov et al., 2003). These latter authors also showed the great conservatism between this rhinoceros species and the white rhinoceros (2n=82) and compared, by chromosome painting, chromosome segments with Burchell's zebra. The X-chromosome is the only large metacentric element but many other elements have small short arms, even the Y-chromosome. Studies of numerous black rhinos from different regions in Africa and known subspecies indicated that all animals possess 2n=84 but that minor differences in heterochromatin exist. Also, an animal biopsy from the Los Angeles Zoo, later transferred to Kansas City where it died, had Trisomy X (CRES unpublished). Allozyme variation was studied in African and Indian rhinoceroses by Merenlender et al. (1989). Kapur et al. (2003) developed DNA satellite tools to differentiate Indian from black rhinos. Microsatellite loci were polymorphic in a study of five black rhinos undertaken by Cunningham et al. (1999). Other studies to distinguish among different black rhinoceros populations were undertaken by Swart et al. (1994) and Ali et al. (1999). Hybrids have not been described.
Ashley et al. (1990) advocated and performed some genetic studies on 23 black rhinos and found only small mtDNA differences, advocating larger surveys for this rapidly declining species. In contrast, Swart et al. (1994) found considerably more variation when they studied protein-coding loci in a very large number of black rhinos from different locations. In a later publication, Swart & Ferguson (1997) also detected differences and regional differences that indicated an east-west cline, and no evidence of inbreeding. Jama et al. (1993) studied aspects of the mtDNA control region.
 |
Karyotype of female black rhinoceros. |
14) Immunology
No studies are known to us, other than serologic surveys. Thus, Anderson & Rowe (1998) found antibodies to Rift Valley Fever (RVF) virus in black rhinos of Zimbabwe . Fischer-Tenhagen et al. (2000) found antibodies to a variety of other viral agents, but not against RVF virus in 281 animals from South Africa , Kenya and Namibia.
15) Pathological features
A major problem for black rhinoceroses is the propensity to develop hemolytic anemia (Chaplin, et al., 1986; Miller & Boever, 1982; Paglia et al., 1986; Paglia, 1993). The major enzymatic causes of this phenomenon were well described by Paglia (1993), and hematologic parameters studied by Chaplin et al. (1986). Veterinary problems, diseases and numerous procedures, were summarized by Silberman & Fulton (1979), and a complete veterinary bibliography was compiled by Miller (1983). It includes references to reproductive laboratory data as well.
Dermal problems and infections appear to be the commonest ailments, including pox (Schaller & Pilaski, 1979; Griner, 1983). Skin ulcers, studied by Kock & Kock (1990) in free-living animals from Zimbabwe were mostly or only due to filarial nematodes ( Stephanofilaria dinniki ). Other parasites have been described; thus, Keirans (1993) found Ixodid ticks in white and black rhinos; babesiasis was reported in three animals from Tanzania (Nijhof et al., 2003); trypanosomiasis was reported by Mihok et al. (1992) and Knapp et al. (1997) collected various arthropods and helminths from black rhinos. Neiffer et al. (2001) described leptospirosis in two black rhinos from Pittsburgh Zoo, presumably acquired from raccoons. Salmonellosis killed two rhinos in Denver (Kenny et al., 1997) and a survey of US zoos (Kenny, 1999) indicated an approximately 10% infection rate.
Three captive black rhinos showed severe encephalomalacia at death whose etiology remains unknown (Miller et al., 1990). Jones (1979) saw ulcerative stomatitis in black rhinos, a lesion later also described by Ott et al. (1982). Pessier et al. (2004) later associated this to eosinophilic granulomas occurring in rhinos but distinguished it from the necrolytic dermatitis described by Munson et al. (1998). Such skin lesions led Grant et al. (2002) to study fatty acids of the foods eaten; diets in the wild were significantly different in their composition. Following prolonged antibiotic therapy of hoof abscesses, a black rhino died from pulmonary aspergillosis (Woods et al., 1999). Acute lymphoblastic leukemia in a 21 months-old animal was the cause of death, perhaps also related to cardiotoxicity of the therapeutic agents (Radcliffe et al., 2000; Paglia & Radcliffe, 2000). That case study suggested excess iron in black rhinos, a notion that was more recently confirmed by additional studies (Paglia et al., 2001). These studies suggested greater prevalence of iron storage in females and suggested a link to the encephalopathy. Stegmann et al. (2001) described the fascinating story of anesthesia and operation of a necrotic rectal prolapse in a free-ranging black rhino. The animal died and was then found to have old pelvic fractures that may have been contributory (Olivier et al., 2001).
16) Physiologic data
Whatever relevant physiologic data are available have been provided in review form by Silberman & Fulton (1979), and as bibliography by Miller (1983). Schaurte (1966) described in detail the various dental formulas and depicts teeth of all rhinocerotidae. Schaffer et al. (1998) studied the reasons for the difficulty of obtaining semen by using sonography. They demonstrated the urethral filling in conditioned animals and the benefits of sonographic usage. O'Brien & Roth (2000) provided a protocol for the conservation and recovery of semen from Sumatran and black rhinos. Meiswinkel (1987) described mosquitoes grown from elephant and rhino dung. Schaffer et al. (2001) found the anatomy and histology of reproductive tracts similar to other species. Maluf (91991; 1994) described the anatomy of the kidney with its 60 lobes. Dierenfeld et al. (1988) found that plasma vitamin E levels of 31 wild animals were significantly higher than in eleven animals of the captive population. In a subsequent study, these results were not verified, presumably because of vitamin supplementation in captive animals (Clauss et al., 2002). Hematologic parameters, especially following the frequent translocations occurring in Africa, were delineated by Kock et al. (1999). The episodic occurrences of hemolysis have led to study particulars of enzymes etc. in rhino red cells. Thus, Weber et al. (2004) found significantly higher tyrosine levels in wild animals compared to captive specimens. For the same reasons, the hemoglobins were studied in several animals and related species (Fairbanks & Miller, 1990). They found considerable polymorphism of black rhino hemoglobins and proposed a genetic mechanism for these proteins.
17) Other resources
Cell strains of four species of rhinoceros are available from the “Frozen zoo” at the Zoological Society of San Diego by contacting Dr. Oliver Ryder at: oryder@ucsd.edu . None are available of the severely endangered Javan rhinoceros. An International Rhino Conference was held at the San Diego Zoo in 1991. It reviews many topics; a copy of the review of this conference can also be obtained from Dr. O. Ryder.
18) Other remarks – What additional Information is needed?
There are no studies on early implantation; information on the structure and length of umbilical cord is needed.
Acknowledgement
The animal photograph in this chapter comes from the Zoological Society of San Diego. The B&W photographs come from R. Montali at the National Zoo, Washington, DC.
References
Ali, S., Azfer, M.A., Bashamboo, A., Mathur, P.K., Malik, P.K., Mathur, V.B., Raha, A.K. and Ansari, S.: Characterization of a species-specific repetitive DNA from a highly endangered wild animal, Rhinoceros unicornis , and assessment of genetic polymorphism by microsatellite associated sequence amplification (MASA). Gene 228:33-42, 1999.
Anderson, E.C. and Rowe, L.W.: The prevalence of antibody to the viruses of bovine virus diarrhea, bovine herpes virus 1, rift valley fever, ephemeral fever and bluetongue and to Leptospira sp. in free-ranging wildlife in Zimbabwe. Epidemiol. Infect. 121:441-449, 1998.
Ashley, M.V., Melnick, D.J. and Western, D.: Conservation genetics of the black rhinoceros (Diceros bicornis), I. : Evidence from the mitochondrial DNA of three populations. Conserv. Biol. 4:71-77, 1990.
Benirschke, K. and Lowenstine, L.J.: The placenta of the rhinocerotidae. Verh. Ber. Erkr. Zootiere (Dresden). 37:15-23, 1995.
Brown, J.L., Bellem, A.C, Fouraker, M., Wildt, D.E and Roth, T.L.: Comparative analysis of gonadal and adrenal activity in the black and white rhinoceros in North America by noninvasive endocrine monitoring. Zoo Biol. 20:463-486 2001.
Chaplin, H. Jr., Malecek, A.C., Miller, R.E., Bell , C.E., Gray, L.S. and Hunter, V.L.: Acute intravascular hemolytic anemia in the black rhinoceros: hematologic and immunologic observations. Amer. J. Vet. Res. 47:13131-1320, 1986.
Clauss, M., Jessup, D.A., Norkus, E.B., Chen, T.C., Holick, M.F., Streich, W.J. and Dierenfeld, E.S.: Fat soluble vitamins in blood and tissues of free-ranging and captive rhinoceros. J. Wildl. Dis. 38:402-413, 2002.
Cunningham, J., Harley, E.H. and O'Ryan, C.: Isolation and characterization of microsatellite loci in black rhinoceros (Diceros bicornis). Electrophoresis 20:1778-1780, 1999.
Dierenfeld, E.S. du Toit, R. and Miller, R.E.: Vitamin E in captive and wild black rhinoceros (Diceros bicornis). J. Wildl. Dis. 24:547-550, 1988.
Fairbanks, V.F. and Miller, R.E.: Beta-globin chain hemoglobin polymorphism and hemoglobin stability in black rhinoceroses (Diceros bicornis). Amer. J. Vet. Res. 51:803-807, 1990.
Fischer-Tenhagen, C., Hamblin, C., Quandt, S. and Frolich, K.: Serosurvey for selected infectious disease agents in free-ranging black and white rhinoceros in Africa . J. Wildl. Dis. 36:316-323, 2000.
Galama, W.T., Graham, L.H. and Savage, A.: Comparison of fecal storage methods for steroid analysis in black rhinoceros (Diceros bicornis). Zoo Biol. 23:291-300, 2004.
Garnier, J.N., Holt, W.V. and Watson, P.F.: Non-invasive assessment of oestrous cycles and evaluation of reproductive seasonality in the female wild black rhinoceros (Diceros bicornis minor). Reproduction 123:877-889, 2002.
Garniera, J.N., Green, D.I., Pickard, A.R., Shaw, H.J. and Holt, W.V.: Non-invasive diagnosis of pregnancy in wild black rhinoceros (Diceros bicornis minor) by faecal steroid analysis. Reprod. Fertil. Dev. 10:451-458, 1998.
George, M. Jr., Chemnick, L.G., Cisova, D., Gabrisova, E., Stratil, A. and Ryder, O.A.: Genetic differentiation of white rhinoceros subspecies: diagnostic differences in mitochondrial DNA and serum proteins. In, Proc. Intern. Conference on Rhinoceros Biology and Conservation, San Diego , CA 1991, pp. 105-113.
Gotch, A.F.: Mammals – Their Latin Names Explained. Blandford Press, Poole , Dorset , 1979.
Grant, J.B., Brown, D.L. and Dierenfeld, E.S.: Essentially fatty acid profiles across diets and browse of black rhinoceros. J. Wildl. Dis. 38:132-142, 2002.
Griner, L.A. : Pathology of Zoo Animals. Zoological Society of San Diego , San Diego , California , 1983.
Groves , C.P.: Phylogeny of the living species of Rhinoceros. Z. zool. System. Evol. 21:293-313, 1983.
Hayssen, V., van Tienhoven, A. and van Tienhoven, A.: Asdell's Patterns of Mammalian Reproduction: a Compendium of Species-specific Data. Comstock/Cornell University Press, Ithaca , 1993.
Hindle, J.E., Mostl, E. and Hodges, J.K.: Measurement of urinary oestrogens and 20 alpha-dihydroprogesterone during ovarian cycles of black (Diceros bicornis) and white (Ceratotherium simum) rhinoceroses. J. Reprod. Fertil. 94:237-249, 1992.
Houck, M.L., Ryder, O.A., Kumamoto , A.T. and Benirschke, K.: Cytogenetics of the rhinocerotidae. Verhandlungsbericht der Erkrankungen der Zootiere. # 37:25-32, 1995). Published by Tierpark Berlin . (This is appended here at the end).
Hungerford, D.A., Chandra, H.S. and Snyder, R.L.: Somatic chromosomes of a black rhinoceros (Diceros bicornis Gray 1821). Amer. Naturalist 101:357-358, 1967.
Jama, M., Zhang, Y., Aman, R.A. and Ryder, O.A.: Sequence of the mitochondrial control region, tRNA Thr , tRNA Pro and tRNA Phe genes from the black rhinoceros, Diceros bicornis . Nucleic Acid Res. 21:4392, 1993.
Jones, D.M.: The husbandry and veterinary care of captive rhinoceroses. Intern. Zoo Ybk. 19:239, 251, 1979.
Kapur, V., Prasanth, S.G., O'Ryan, C., Azfer, M.A. and Ali, S.: Development of a DNA marker by minisatellite associated sequence amplification (MASA) from the endangered Indian rhino (Rhinoceros unicornis). Mol. Cell Probes 17:1-4, 2003.
Keirans, J.E.: Dermatocentor rhinocerinus (Denny 1843) (Acari: Ixodida:ixodidae): redescription of the male, female and nymph and first description of the larva. Onderstepoort J. Vet. Res. 60:59-68, 1993.
Kenny, D.E.: Salmonella sp. Survey of captive rhinoceroses in U.S. zoological institutions and private ranches. J. Zoo Wildl. Med. 30:383-388, 1999.
Kenny, D.E., Baier, J. and Getzy, D.M.: Salmonellosis in captive black rhinoceros (Diceros bicornis). J. Zoo Wildl. Med. 28:307-311, 1997.
Knapp, S.E., Krecek, R.C., Horak, I.G. and Penzhorn, B.L.: Helminths and arthropods of black and white rhinoceroses in southern Africa . J. Wildl. Dis. 33:492-502, 1997.
Kock, N. and Kock, M.D.: Skin lesions in free-ranging black rhinoceroses (Diceros bicornis) in Zimbabwe . J. Zoo Wildl. Med. 21:447-452, 1990.
Kock, N., Morton, D. and Kock, M.: Reproductive parameters in free-ranging female black rhinoceroses (Diceros bicornis) in Zimbabwe . Onderstepoort J. Vet. Res. 58:55-57, 1991.
Kock, R.A., Mihok, S.R., Wambua, J., Mwanzia, J. and Saigawa, K.: Effects of translocation on hematologic parameters of free-ranging black rhinoceros (Diceros bicornis michaeli) in Kenya . J. Zoo Wildl. Med. 30:389-396, 1999.
Lance, V.A., Patton, M.L. and Hagey, L.R.: Identification of a series of C(21)O(2) pregnanes from fecal extracts of a pregnant black rhinoceros (Diceros bicornis). Steroids 66:875-881, 2001.
Maluf , N.S. : Renal morphology of the hook-lipped African rhinoceros, Diceros bicornis , Linnaeus. Amer. J. Anat. 190:245-265, 1991.
Maluf. N.S. Further studies on the kidney of the hook-lipped African rhinoceros, Diceros bicornis . Anat. Rec. 238:38-48, 1994.
Meiswinkel, R.: Afrotropical culicoides: a redescription of C. (Avaritia) kanagai Khamala & Kette, 1971, reared from elephant dung in the Kruger National Park, South Africa). Onderstepoort J. Vet. Res. 54:585-590, 1987.
Merenlender, A.M., Woodruff, D.S., Ryder, O.A., Kock, R. and Váhala, J.: Allozyme variation and differentiation in African and Indian rhinoceroses. J. Hered. 80:377-382, 1989.
Mihok, S., Olubayo, R.O. and Moloo, S.K.: Trypanosomiasis in the black rhinoceros (Diceros bicornis Linnaeus, 1758). Rev. Sci. Tech. 11:1169-1173, 1992.
Miller, R.E.: Veterinary Bibliography for Rhinoceros. A.A. Balkema Publ., Amsterdam, 1983.
Miller, R.E. and Boever, W.J.: Fatal hemolytic anemia in the black rhinoceros: Case report and a survey. J.A.V.M.A. 181:1228-1231, 1982.
Miller, R.E., Cambre, R.C., de Lahunta, A., Brannian, R.E., Spraker, T.R., Johnson, C. and Boever, W.J.: Encephalomalacia in three black rhinoceroses (Diceros bicornis). J. Zoo Wildl. Med. 21:192-199, 1990.
Morales, J.C. and Melnick, D.J.: Molecular systematics of the living rhinoceros. Mol. Phylogenet. Evol. 3:128-134, 1994.
Munson, L., Koehler, J.W., Wilkinson, J.E. and Miller, R.E.: Vesicular and ulcerative dermopathy resembling superficial necrolytic dermatitis in captive black rhinoceroses ( Diceros bicornis ). Vet. Pathol. 35:31-42, 1998.
Neiffer, D.L., Klein, E.C. and Wallace-Switalski, C.: Leptospira infection in two black rhinoceroses (Diceros bicornis). J. Zoo Wildl. Med. 32:476-486, 2001.
Nijhof, A.M., Penzhorn, B.L., Lynen, G., Mollel, J.O., Morkel, P., Bekker, C.P. and Jongejan, F.: Babesia bicornis sp. Nov. and Theileria bicornis sp. Nov.: tick-borne parasites associated with mortality in the black rhinoceros (Diceros bicornis). J. Clin. Microbiol. 41:2249-2254, 2003.
O'Brien, J.K. and Roth, T.L.: Post-coital sperm recovery and cryopreservation in the Sumatran rhinoceros (Dicerorhinus sumatrensis) and application to gamete rescue in the African black rhinoceros (Diceros bicornis). J. Reprod. Fertil. 118:263-271, 2000.
Olivier, A., Lane, E., Volkmann, D.H., Hofmeyr, M. and Stegmann, G.F.: Rectal prolapse associated with a healed pelvic fracture in a pregnant free-ranging African black rhinoceros (Diceros bicornis). Part 2: surgery and necropsy. J. S. Afr. Vet Assoc. 72:242-244, 2001.
Ott, J. E., McDonald, S.E., Robinson, P.T. and Wright, F.H.: Ulcerative stomatitis in a black rhinoceros (Diceros bicornis). AAZV Ann. Proceed. 68-71, 1982.
Paglia, D.E.: Acute episodic hemolysis in the African black rhinoceros as an analogue of human glucose-6-phosphate dehydrogenase deficiency. Amer. J. Hematol. 42:36-45, 1993.
Paglia, D.E. and Radcliffe, R.W.: Anthracycline cardiotoxicity in a black rhinoceros (Diceros bicornis): evidence for impaired antioxidant capacity compounded by iron overload. Vet. Pathol. 37:86-88, 2000.
Paglia, D.E., Valentine, W.N., Miller, R.E., Nakatani, M. and Brockway, R.A.: Acute intravascular hemolysis in the black rhinoceros: Erythrocyte enzymes and metabolic intermediates. Amer. J. Vet. Res. 47:1321-1325, 1986.
Paglia, D.E., Kenny, D.E., Dierenfeld, E.S. and Tsu, I.H.: Role of excessive maternal iron in the pathogenesis of congenital leukoencephalomalacia in captive black rhinoceroses (Diceros bicornis). Amer. J. Vet. Res. 62:343-349, 2001.
Pessier, A.P., Munson, L. and Miller, R.E.: Oral, nasal, and cutaneous eosinophilic granulomas in the black rhinoceros (Diceros bicornis): a lesion distinct from superficial necrolytic dermatitis. J. Zoo Wildl. Med. 35:1-7, 2004.
Radcliffe, R.W., Paglia, D.E. and Couto, C.G.: Acute lymphoblastic leukemia in a juvenile southern black rhinoceros (Diceros bicornis). J. Zoo Wildl. Med. 31:71-76, 2000.
Radcliffe, R.W., Eyres, A.I., Patton, M.L., Czekala , N.M. and Emslie, R.H.: Ultrasonographic characterization of ovarian events and fetal gestational parameters in two southern black rhinoceros (Diceros bicornis) and correlation to fecal progesterone. Theriogenology 55:1033-1049, 2001.
Ryder, O.A., ed.: Rhinoceros Biology and Conservation. Zoological Society of San Diego , 1993 (Proc. of Conference 1991).
Schaffer, N., Bryant, W., Agnew, D., Meehan, T. and Beehler, B.: Ultrasonographic monitoring of artificially stimulated ejaculation in three rhinoceros species (Ceratotherium simum, Diceros bicornis, Rhinoceros unicornis). J. Zoo Wild. Med. 29:386-393, 1998.
Schaffer, N.E., Foley, G.L., Gill, S. and Pope, C.E.: Clinical implications of rhinoceros reproductive tract anatomy and histology. J. Zoo Wildl. Med. 32:31-46, 2001.
Schaurte, W.T.: Beiträge zur Kenntnis des Gebisses und Zahnbaues der afrikanischen Nashörner. Säugetierkundl. Mitt. 14:327-341, 1966.
Schwarzenberger, F., Francke, R. and Goltenboth, R.: Concentration of faecal immunoreactive progestagen metabolites during the oestrous cycle and pregnancy in the black rhinoceros (Diceros bicornis). J. Reprod. Fertil. 98:285-291, 1993.
Silberman, M.S. and Fulton , R.B.: Medical problems of captive and wild rhinoceros – a review of the literature and personal experiences. J. Zoo Anim. Med. 10:6-16, 1979.
Stegmann, G.F., Hofmeyr, M., Olivier, A., Lane, E. and Volkmann, D.H.: Rectal prolapse associated with a healed pelvic fracture in a pregnant free-ranging African black rhinoceros (Diceros bicornis). Part 1: anaesthesia. J. S. Afr. Vet. Assoc. 72:239-241, 2001.
Swart, M.K., Ferguson , J.W., du Toit, R. and Flamand, J.R.: Substantial genetic variation in southern African black rhinoceros (Diceros bicornis). J. Hered. 85:261-266, 1994.
Swart, M.K.J. and Ferguson , J.W.H.: Conservation implications of genetic differentiation in Southern African populations of black rhinoceros (Diceros bicornis). Conserv. Biol. 11:79-83, 1997.
Tougard, C., Delefosse, T., Hanni, C. and Montgelard, C.: Phylogenetic relationships of the five extant Rhinoceros species (Rhinocerotidae, Perissodactyla) based on mitochondrial cytochrome b and 12S rRNA genes. Mol. Phylogenet. Evol. 19:34-44, 2001.
Trifonov, V., Yang, F., Ferguson-Smith, M.A. and Robinson, T.J.: Cross-species chromosome painting in the Perissodactyla: delimitation of homologous regions in Burchell's zebra (Equus burchellii) and the white (Ceratotherium simum) and black rhinoceros (Diceros bicornis). Cytogenet. Genome Res. 103:104-110, 2003.
Turner, J.W. Jr., Tolson, P. and Hamad, N.: Remote assessment of stress in white rhinoceros (Ceratotherium simum) and black rhinoceros (Diceros bicornis). J. Zoo Wildl. Med. 33:214-221, 2002.
Weber, B.W., Paglia, D.E. and Harley, E.H.: Elevated free tyrosine in rhinoceros erythrocytes. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 138:105-109, 2004.
Woods, R., Blyde, D.J., Seaman, J.T. and Thorne, A.H.: Fungal pneumonia in a captive black rhinoceros. Austral. Vet. J. 77:717-719, 1999.
ADDITION
From: Verhandlungsberichte der Erkrankungen der Zootiere (# 37, 25-32, 1995)
From the Center for Reproduction of Endangered Species, Zoological Society of San Diego, California and the Department of Pathology, University of California , San Diego
CYTOGENETICS OF THE RHINOCEROTIDAE
By Marlys L. Houck, 0. A. Ryder, Arlene T. Kumamoto and K. Benirschke
Introduction
The family Rhinocerotidae is comprised of five extant species. Ceratotherium simum (white rhinoceros) and Diceros bicomis (black rhinoceros) are found in Africa , while Dicerorhinus sumatrensis (Sumatran rhinoceros), Rhinoceros unicomis (Indian rhinoceros) and Rhinoceros sondaicus (Javan rhinoceros) are found in Asia . Rhinoceros populations in both Africa and Asia have declined rapidly in recent decades due to loss of habitat and poaching for the horn (PENNY, 1988). The 1994 IUCN Red List of Threatened Animals, (GROOMBRIDGE, 1993), lists four of the species as endangered and C. simum as vulnerable.
In spite of intensive conservation interest in all five species of the Rhinocerotidae, little is known about the cytogenetics of this family. Although several cytogenetic studies have been conducted on three of the five species, the sample sizes have been small and the results conflicting. The diploid number of two of the species, D. sumatrensis and R. sondaicus, has not been previously documented. Early reports on the chromosomes of C. simum gave conflicting diploid numbers of 82 or 84 based on studies involving only a few specimens (HANSEN, 1976; HEINICHEN, 1967, 1970; HSU and BENIRSCHKE, 1973). The diploid number of D. bicomis was reported as 2n = 84 by HUNGERFORD et al (1967) and HEINICHEN (1970), and at that time was the highest diploid number known for mammals. R. unicornis was documented as 2n = 82 by WURSTER and BENIRSCHKE (1968) and HSU and BENIRSCHKE (1973). More recently, preliminary information on the chromosomes of C. simum, D. bicomis, D. sumatrensis and R. unicornis was reported by RYDER et al (1987).
Little data exists on chromosome banding in rhinoceros species. Initially, one partially Q-banded karyotype of C. simum was presented by HANSEN (1976). Recently, the first comprehensive chromosome banding studies of Ceratotherium were published including the first chromosomal studies of the northern white rhinoceros (C. s. cottoni) (HOUCK et al, 1993). Here, we present for the first time the diploid chromosome number of D. sumatrensis as well as the first chromosomal banding studies of D. bicomis, R. unicomis and D. sumatrensis.
Materials and methods
Cytogenetic studies were conducted on 113 rhinoceroses. These included 25 male and 35 female Diceros bicornis, 12 male and 27 female C. simum, five male and three female R. unicornis , and one male and five female D. sumatrensis. A summary of the studbook numbers and institutions of the animals included in this study is presented in Table 1. All samples were acquired from captive populations with the exception of 11 D. bicomis samples which were obtained from wild populations in Zimbabwe . Metaphase chromosomes were obtained from fibroblast and/or lymphocyte cultures.
Skin biopsies from 11 of the D. bicomis individuals were obtained under field conditions in Zimbabwe during the rhinoceros dehorning project conducted by the Zimbabwe Department of National Parks and Wildlife Management. Small epidermal skin samples were taken from the axillary region of anesthetized animals. Field conditions require extreme caution in specimen handling to avoid contamination. The samples were minced into 1 mm 3 fragments and placed in cryovials containing 1 ml of freeze medium. The freeze medium consisted of supplemented a-MEM (described below) with 10% dimethyl sulfoxide. The cryovials were placed into a primed liquid nitrogen dry shipper and transported to the laboratory in San Diego . To initiate fibroblast cultures the samples were quick-thawed at 37°C and digested in collagenase.
INSTITUTION |
STUOBOOK NUMBER |
|||
Atlanta Zoo, GA |
|
38 335 |
|
|
Audubon Park Zoo, LA |
24 579 580 |
|
|
|
Busch Gardens , FL |
45 |
343 |
|
|
Cincinnati Zoo, OH |
417 418 |
180 |
|
|
Dallas Zoo, TX |
|
67 |
|
|
DenverZoo,C0 |
|
161 163 328 332 432 *a |
|
|
Detroit Zoo, Ml |
|
55 281 409 |
|
|
Dvur Kralove Zoo, Czech Republic |
351 372 374 |
|
|
|
Fossil Rim Wldlf Cent., TX |
|
470 |
|
|
Granby Zoo , Canada |
|
293 |
|
|
Henry Vilas Zoo, WI |
696 |
|
|
|
Kansas City Zoo, MO |
|
360 |
|
|
Kings Dominion WAP, VA |
751 |
|
|
|
Knoxville Zoo, TN |
452 |
|
|
|
Köln Zoo , Germany |
|
|
|
84 |
Lee Richardson Zoo, KS |
|
124 125 |
|
|
Los Angeles Zoo, CA |
|
267 333 334 |
|
|
Melaka Zoo, Malaysia |
|
|
1 19 *b |
|
Miami MetroZoo, FL |
|
52 |
|
*c |
Milwaukee Zoo, WI |
|
|
|
14 |
Oklahoma City Zoo, OK |
|
196 330 |
|
|
Port Lympne Zoo Park, UK |
|
|
10 |
|
RiverbanksZoo , SC |
|
381 383 |
|
|
San Antonio Zoo, TX |
182 897 |
|
|
|
San Diego Wild Animal Park , CA |
28 49 74 |
110 179 239 302 427 435 |
|
9 78 143 *d |
San DiegoZoo, CA |
|
78 188 390 392 473 SD1 |
28 33 |
|
San Francisco Zoo, CA |
|
74 351 |
|
|
Sedgwick County Zoo, KS |
|
53 192 |
|
|
St. LouisZoo , MO |
|
121 212 251 |
|
|
White Oak Plantation, FL |
|
471 |
|
|
Zimbabwe |
|
*f |
|
|
*b Female, “Tengagoh”, studbook number unknown
*c Female, stillborn 4/91
*d Male, stillborn 2/92
*e Male, stillborn 11/92
*f Eleven samples were collected in July 1993 during the Zimbabwe Department of National Parks and Wildlife Management rhinoceros dehorning project.
Fibroblast cultures were established from skin biopsies by tissue dissociation in 0.5% collagenase (Boehringer Mannheim). This technique usually yields fibroblast attachment in a T25 flask within 24-48 hours. This represents a considerable improvement over the previously used explant method which typically required two -three weeks before fibroblast growth appeared. Cultures were maintained in a 1:1 mixture of Minimal Essential Medium Alpha (a-MEM; GIBCO BRL) and Fibroblast Growth Medium (FGM; Clonetics). The a-MEM was supplemented with 10% fetal bovine serum (FBS), 1% glutamine and 1% penicillin-streptomycin. The human fibroblast growth factor and insulin in the FGM medium is believed to have enhanced the growth and normal cell division of the cultures over that of cells grown in supplemented a-MEM alone. Cells were harvested using actinomycin D (Cosmegen, Merck Sharp and Dohme) and Velban (Eli Lily and Company) following the method described by YU et al. (1981) with the modification of a 30 minute hypotonic incubation at 37°C in 0.075 M KCI. For lymphocyte cultures a cell suspension of autologous plasma and buffy coat (ap/bc) was obtained from 10 ml of heparinized blood according to the method described by RYBAK et al. (1982). Lymphocyte cultures were initiated as soon as possible after sampling since the mitotic index at harvest decreased substantially with the age of the sample. Cultures initiated from blood samples over two days old did not usually yield chromosomes. Cultures containing 9.5 ml of supplemented a-MEM medium and 0.5 - 1.0 ml of ap/bc were incubated with 0.3 ml pokeweed mitogen (Gibco BRL) and 6 ug/ml phorbol 12-myristate 13-acetate 4-0 methyl ether (Sigma) comitogen. Following a 114 hour incubation period at 37°C cells were harvested as above.
The chromosomes of all 113 animals were examined by non-differential staining using Giemsa. C-banding followed the method described by SUMNER (1972), and G-banding was done according to the method of SEABRIGHT (1971). Chromosomes were aligned in karyotypes by decreasing size, with submetacentric pairs presented first. Because of the difficulty in obtaining diagnostic G-bands, there was no attempt to standardize similar chromosomal pairs among the four species. High resolution banding attempts were unsuccessful due to problems obtaining banding-quality metaphases when dealing with such a high number of mainly acrocentric chromosomes.
Results and discussion
Non-differentially stained karyotypes were completed on all 113 samples. The modal diploid chromosome number for the four species examined was 2n 82 for C. simum, D. sumatrensis and R. unicornis and 2n = 84 for D. bicomis . Nothing is known about the chromosomes of the rare Javan rhinoceros, R. sondaicus . A comparison of modal diploid numbers, heterochromatic regions, and morphology of the autosomes and sex chromosomes of the four species are shown in Table 2.
Table 2: Summary of modal diploid numbers, morphology and heterochromatin of the four rhinoceros species studied
|
n |
MODAL |
AUTOSOMES |
X |
V |
HETEROCHROMATIC |
Ceratotherium simum |
36 |
82 |
80 t and a |
large sm |
small sm |
centromeres |
Diceros bicomis |
|
84 |
82 Sm, t and a |
large sm |
small m |
centromeres |
Dicerorhinus |
6
8 |
82 |
80 a |
large sm |
small a? |
most centromeres |
Rhinoceros unicomis |
|
82 |
16 to 20 sm |
large sm |
small a? |
centromeres |
a = acrocentric, t = telocentric, sm = submetacentric, m = metacentric
Although detailed Q-band comparisons among the four species were not conducted, the dissimilar morphology and lack of Q-banding similarities found among the largest chromosomal pairs in each species suggest that the differences in diploid number involve more than a mere fission event of one bi-armed chromosomal pair. Morphological and molecular analyses place the two African rhinoceros species as each others closest relatives with the Asian rhinos as a sister group (GROVES, 1983; PROTHERO, 1986; AMATO, et al., 1993; but see MORALES and MELNICK, 1994). Accordingly, the change in diploid number within African rhinoceroses would most parsimoniously be considered to have taken place after the divergence of Diceros and Ceratotherium from a common ancestor possessing 2n=82, an apparently ancestral condition in the Rhinocerotidae.

Fig. 1 : G-banded karyotype of a female black rhinoceros, 2n = 84. Arrows indicate p-arm po!ymorphisms.
Black rhinoceros
Karyotype analyses of D. bicomis revealed 82 submetacentric, telocentric and acrocentric autosomes and the two sex chromosomes. The number of bi-armed chromosomes and size of the short arms (p-arms) varied among individuals. These results are consistent with previously published chromosomal data (HEINICHEN, 1970; HUNGERFORDI 1967). C-banding showed that the p-arms were largely heterochromatic. Centromeric heterochromatin was present on all elements, and interstitial C-bands were observed on one or two of the autosomes. 0-banding revealed size polymorphisms involving short arm additions in many chromosomal pairs (Figure 1). A bimodal distribution of the number of autosomes with heterochromatic p-arms differentiated the eastern and southern populations of black rhinoceroses. These data, which are consistent with recent limitation of gene flow between these regional populations, will be presented elsewhere.
White rhinoceros
The normal karyotype of C. simum consists of 80 telocentric and acrocentric autosomes and the two sex chromosomes. Centromeric heterochromatin was present on all elements (HOUCK et al, 1993). These findings are in agreement with the diploid number of 82 reported by HEINICHEN (1967,1970). The conflicting reports of 2n = 84 (HANSEN, 1976; HSU and BENIRSCHKE, 1973) can be attributed to their small sample sizes and the difficulty of scoring the high number of very small acrocentric elements.
Indian Rhinoceros
The autosomes of R. unicomis consisted of 16 - 20 submetacentric chromosomes and the remaining elements were acrocentric. The diploid number was 82, which is consistent with previously published karyotypes for this species (WURSTER and BENIRSCHKE, 1968; HSU and BENIRSCHKE, 1973). As in D. bicomis, 0-banding revealed short arm polymorphisms which made it difficult to determine a consistent number of bi-armed pairs (Figure 2). C-banded karyotypes showed that the short arms were largely heterochromatic and all elements contained centromeric heterochromatin. One or two autosomal elements also had regions of interstitial heterochromatin (Figure 3).
Sumatran rhinoceros
The normal karyotype of D. sumatrensis consisted of 80 acrocentric autosomes of decreasing size and the two sex chromosomes. C-banding revealed centromeric heterochromatin on most elements as well as interstitial bands on one or two autosomal pairs and a large block of heterochromatin near the terminal end of the long arm (q-arm) of the X chromosome (Figure 4). A size polymorphism in the G-band negative area of the second largest chromosome pair was observed in one of the two individuals that were G-banded. The karyotype of the animal with the normal pair 2 is shown here (Figure 5).
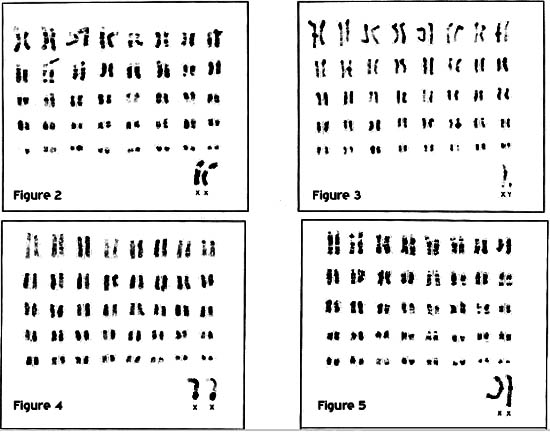
Fig. 2 : G-banded karyotype of a female Indian rhinoceros, 2n = 82. Arrow indicates p-arm polymorphism.
Fig. 3 : C-banded karyotype of a male Indian rhinoceros, 2n = 82.
Fig. 4 : C-banded karyotype of a female Sumatran rhinoceros, 2n = 82, showing the addition of heterochromatin near the q terminal! ends of the X chromosomes.
Fig. 5 : G-banded karyotype of a female Sumatran rhinoceros, 2n = 82.
The X chromosomes of C. simum, D. bicomis and R. unicomis were identical. D. sumatrensis, however, had additional heterochromatic material near the Xq terminal end. In all four species the X was a large submetacentric element having the largest p-arm of any of the elements. With an arm ratio of 1.4, the X chromosomes in D. bicomis and R. unicomis were easily distinguished from large submetacentric autosomes which consistently had arm ratios of 2. In D. sumatrensis, the arm ratio of the X chromosome was 2.6, but it was distinct in this species because it was the only submetacentric element in the karyotype. Except for D. sumatrensis , the G-band pattern of the rhinoceros X chromosome was similar to the submetacentric form of the “original or standard type” found in other mammalian species including humans (OHNO, 1967; PATHAK and STOCK, 1974). The banding pattern of the X chromosome of D. sumatrensis was similar to that of the other three rhinoceros species with the addition of several bands at the distal portion of the q-arm where the constitutive heterochromatin was observed. Using the standardized banding nomenclature for the human X chromosome, the insertion occurs at Xq27 (PARIS CONFERENCE, 1972). The distinct G- and C-banding patterns found in the X chromosome of D. sumatrensis have also been observed in the mountain tapir, Tapirus pinchaque (M.L. Houck, unpublished data). Because of the difficulty in obtaining diagnostic 0-bands on small elements it was not possible to distinguish the Y chromosome with confidence in any of the four species. The Y chromosome is probably represented by a small metacentric or submetacentric element in C. simum and D. bicomis and a small acrocentric in D. sumatrensis and R. unicomis . It was not heterochromatic.
Several abnormal karyotypes were found during this study and are summarized in Table 3. None of these animals had congenital anomalies. Karyotypic analyses of three C. s cottoni individuals, a male (#372), and his two female offspring (#789 & #943), revealed a diploid number of 2n = 81 in which two fewer acrocentric chromosomes and one additional metacentric chromosome were observed. The difference between the karyotypes of these individuals and the other 36 specimens of C. simum examined can be accounted for by a Robertsonian translocation (centromere-centromere) mechanism (HOUCK et al, 1993).
Table 3: Abnormal chromosomal complements
|
STDBK # |
2n |
SEX |
ABNORMALITY |
Ceratotherium simum |
372
789 |
81 |
M |
Robertsonian translocation |
Diceros bicornis |
188 |
84 |
F |
Heterochromatic arm addition |
One female D. bicomis individual, (#360), was found to have a diploid number of 2n 85 because of an additional large submetacentric chromosome. Analysis of the 0-bands revealed a trisomy X chromosomal complement. Presumably the additional X did not have any deleterious effects because of X inactivation (LYON , 1962). This animal died at age 2.5 years. Autopsy findings gave no indications of anomalies or untoward effects of trisomy X. Diffuse encephalopathy was deemed to be the result of an unknown toxin or nutritional deficiency (Eric Miller, personal communication). The dam, (#267), of this individual was sampled and revealed a normal 2n 84 karyotype. The sire was not sampled directly, but karyotype analyses of his paternal grandparents, (#124 and #125), revealed normal chromosomal complements.
Two other female D. bicomis individuals, that were wild-caught and presumably unrelated, had normal diploid numbers, but showed very large heterochromatic areas in the p-arm of one chromosome. In one animal, (#293), the additional heterochromatin resulted in a submetacentric element similar in size to the X chromosomes. Due to the quality of the G-banding it was not possible to determine which chromosome was affected. In the other animal, (#188), a metacentric chromosome was created by the addition of a large heterochromatic arm. Q-banding showed a metacentric chromosome in which the q-arm paired with that of a subtelocentric autosome.
This study illustrates the importance of chromosomal analyses in support of animal health and conservation management programs. Further comparative studies need to be conducted on chromosomal polymorphisms which may distinguish different geographic populations of D. bicomis . In addition, the numerical polymorphism noted in the critically endangered northern white rhinoceros ( C. s. cottoni ) should be monitored through additional study of captive specimens and, where feasible, of wild populations.
Acknowledgements
The authors wish to thank Ken Kelley, Grace Magee, Suellen Charter and Reneê Cabrera for their assistance and cooperation. Appreciation is extended to Steve Kingswood, Dr. Valentine L. Vance and Dr. E. Ann 0akenfull for their critical review of the manuscript and to Dr. Mike H. Jurke for translation of the summary. Clonetics Corporation generously provided the necessary growth medium for the fibroblast cultures.
Summary
Cytogenetics of the Rhinocerotidae
Cytogenetic studies were conducted on 113 rhinoceroses, representing four of the five extant species. Karyotype analyses revealed a modal diploid number of 82 chromosomes for Ceratotherium simum, Rhinoceros unicomis, Dicerorhinus sumatrensis and 84 chromosomes for Diceros bicomis . Comparison of the autosomes of the four species revealed differences in centromere location, ranging from all acrocentric elements in D. sumatrensis to mostly submetacentric elements in D. bicomis. The X chromosome was identified as a large submetacentric element in all four species. The X chromosome of D. sumatrensis was distinct from the other three species because of an addition of heterochromatic material in the long arm.
Abnormal chromosomal complements were identified in three C. simum and three D. bicomis including one trisomy X individual. The diploid number of D. sumatrensis is presented for the first time, as well as the first banding studies of D. sumatrensis, R. unicornis and D. bicornis.
Zusammenfassung
CYTOGENETIK DER RHINOCEROTIDAE
Wir berichten über cytogenetische Untersuchungen an 113 Nashömem von vier der fünf bestehenden Nashomarten. Die Chromosomenzahl (“diploide Zahl" “2n”) beträgt 82 Chromosomen in den folgenden drei Arten: Ceratotherium simum, Rhinoceros unicornis und Dicerorhinus sumatrensis. Hingegen hat Diceros bicomis 84 Chromosomen. Wenn man die Autosomen dieser Arten vergleicht, so findet man eine unterschiedliche Lokalisation der Zentromeren, von ausschliesslich akrozentrischen Autosomen in D. sumatrensis zu hauptsächlich submetazentrischen E!ementen im D. bicomis. Das X-Chromosom ist ein grosses submetazentrisches Element in allen Arten. Das X-Chromosom von D. sumatrensis unterscheidet sich allerdings von den anderen Arten durch ein zusätzliches Stück von Heterochromatin im langen Arm. Chromosomenabnormalitäten wurden in je drei Nashömem von D. simum und von D. bicornis gefunden, einschliesslich einer Trisomie des X-Chromosoms. Dieser Bericht stellt die erste Beschreibung der diploiden Chromosomenzahl des D. sumatrensis dar; ebenso präsentieren wir die ersten gebandeten Chromosomen von D. sumatrensis, R. unicomis und von D. bicomis.
Résumé
La cytogénetique des rhinocérotidae
Nous avons effectué des examens cytogénétiques sur 113 rhinoceros ayant represente quatre des cinq espèces existantes. Le nombre de chromosomes (“chiffre diploide" "2n”) se chiffre a 82 chromosomes pour les trois espèces qui suivent: Ceratotherium simum, Rhinoceros unicornis et Dicerorhinus sumatrensis . Diceros bicomis par contre, a 84 XX chromosomes. La comparaison des autosomes de ces espèces révèle une localisation differente des centromères , a partir d'autosomes exclusivement acrocentriques dans l'espèce D. sumatrensis jusqu'aux elements essentiellement submétacentriques dans D. bicomis . Le chromosome X est un grand élément submetacentrique dans toutes les espèces. Cependant, le chromosome X de D. sumatrensis se distingue des autres espèces par un élement additionnel d'héterochromatine au bras long. Nous avons observe des anomalies de chromosomes dans trois specimens de C. simum et dans trois de D. bicornis ainsi qu'une trisomie du chromosome X. Ce rapport présente pour Ia premiere fois une description du chiffre diploide des chromosomes de D. sumatrensis et fait etat des bandes chromosomales de D. sumatrensis, de R. unicomis et de D. bicomis.
References
AMATO, G.D:, ASHLEY, M. and J. GATESY (1993): In, Rhinoceros biology and conservation (O. A. Ryder, ed). Zoological Society of San Diego , San Diego , 114-122.
HANSEN, KM. (1976): Q-bands of some chromosomes of the white rhinoceros. Hereditas 82, 205-208.
HEINICHEN, I. G. (1967): Karyotype of Ceratotherium simum s/mum and Equus zebra zebra. A preliminary note. J. S. Afr. Vet. Med. Assoc.38 247-248.
HEINICHEN, I.G. (1970): Karyologica/ studies on southern African Perissodactyla. Koedoe 13, 51-108.
HOUCK, M.L., RYDER, O.A., VAJALA, J., KOCK, R.A., and J.E. OOSTERHUIS (1993): Diploid chromosome number and chromosomal variation in the white rhinoceros (Ceratotherium simum). J. Hered. 85, 30-34.
HSU, T.C. and K. BENIRSCHKE (1973): An atlas of mammalian chromosomes. Vol. 7. Berlin : Springer- Ver/ag: folios 339-340.
HUNGERFORD, D.A., CHANDRA, H.S., and R.L. SNYDER (1967): Somatic chromosomes of a black rhinoceros (Diceros b/corn/s Gray 1821). Am. Nat. 101, 357-358.
GROOMBRIDGE, B., (Ed.) (1993) 1994 IUCN red list of threatened animals. !UCN Gland , Switzerland and Cambridge , UK .
GROVES , C.P. (1983): Phylogeny of the living species of rhinoceros. Z. Zoo!. Syst. Evolutionforsch. 21, 293-313.
LYON , M.F. (1962): Sex chromatin and gene action in the mammalian X-chromosome. Am. J. Hum. Genet. 14, 135-148.
MORALES, J.C. and D.J. MELNICK (1994): Molecular systematics of the living rhinoceros. Molec. Phylogen. and Evol. 128-134. OHNO, S. (1967): Sex chromosomes and sex-linked genes., Berlin/Heidelberg/ New York : Springer-Verlag.
PARIS CONFERENCE (1972): Standardization in human cytogenetics. Birth defects: Original art ser. VIII, No. 7. The National Foundation, New York .
PATHAK, Sand. D. STOCK (1974): The X chromosome of mammals: Karyological homology as revealed by banding techniques. Genetics 7_8, 703-714.
PENNY, M. (1988): Rhinos: Endangered Species. Facts on File Publications, New York .
PROTHERO, D.R., MANNING, E. and C.B. HANSSON (1986): The phylogeny of the Rhinocerotoidea (Mammalia, Perissodactyla.) Zoo! J. Linn. Soc. 87, 341-366.
RYBAK, J., THARAPEL, A., ROBINETT, S., GARCIA, M., MARK!NEN C., and M. FREEMAN (1982): A simple reproducible method for prometaphase chromosome analysis. Hum. Gen. 60, 328-333.
RYDER, O.A., HOUCK, M.L., and A.T. KUMAMOTO (1987): Rhinoceros genetics: the state-of-the-art and application to conservation measures. American Association of Zoological Parks Annual Proceedings, 710-714.
SEABRIGHT, M. (1971): A rapid technique for human chromosomes. Lancet 2, 971-9 72.
SUMNER, A. T. (1972): A simple technique for demonstrating centromeric heterochromatin. Exp. Cell Res. L5, 304-306.
WURSTER, D.H. and K. BENIRSCHKE (1968): The chromosomes of the great Indian rhinoceros. Experientia 24. 511.
YU, R.L., ARONSON, M.M. and WW. NICHOLS (1981): High-resolution bands in human fibroblast chromosomes induced by actinomycin D. Cytogenet. Cell Genet. 31, 111-114.
Address of authors: Marlys L. Houck, Center for Reproduction of Endangered Species, Zoological Society of San Diego , P. 0. Box 551 , San Diego , C.A. 92112-0551 (USA).