| (Clicking
on the thumbnail images will launch a new window and a larger version
of the thumbnail.) |
| Last
updated: December 14, 2003. |
Daubentonia madagascariensis
Order: Primates
Family: Daubentoniidae
1) General Zoological Data
This single surviving species of this genus inhabits Eastern Madagascar (Malagasy Republic) and weighs between 2 and 2.8 kg (Nowak, 1999). It is now protected and is rarely seen in zoological parks (Paris, Jersey) because of the danger of its extinction, but the animal has been bred in zoos many times (Jones, 1986). There is an interesting description of habitat and the local lore concerning aye-ayes in Jolly's account (1988). It also discusses the unusual digits ("Fingertier") that enable the animal to peck for insect larvae. Aspects of captive management have been described by Pollock (1986) and in Tattersall's book (1982). The name for the genus derives from the French zoologist Louis J.M. Daubenton (Gotch1979). The species was formerly called Cheiromys (Chiromys); and aiay is a Malagasy designation.
Numerous studies have been conducted to ascertain the phylogeny of these primitive primates (Pastorini et al., 2002, and references in the section on genetics below). It is commonly assumed that aye-ayes have split off the primate tree very early and that, subsequently, many chromosomal rearrangements have occurred, especially fissions. In my opinion, this is not necessarily justified. Another possibility exists, that from an originally much larger number of chromosomes, over the very long period since its ancestral "offspringing", numerous fusions have occurred in this genus, thus leading to the low chromosome number and that the intermediates have become extinct. As to the extinction of ancestors or relatives, this issue has also been much debated, as for instance by Groves (2001) and Antonius (2003) without there being a clear resolution.
The Duke University Primate Center has bred some of these animals recently and the specimen described here comes from that facility. This is a solitary, nocturnal species with frugivorous and insectivorous diet and complex nest building. A captive Aye-aye lived to 23 years. Feistner & Ashbourne (1994) described in some detail the development for the first year of an aye-aye born in captivity.
 |
Aye-aye at Duke University Primate Center |
 |
Aye-aye at Duke University Primate Center. |
Single young are produced. The only placenta available to me is the one depicted below. After formalin fixation the placenta weighed 2.8 g, measured 3.5 x 2.5 x 0.3 cm and had a marginally inserted 8.5 cm umbilical cord. The placenta was uniformly thin and brown, with a slightly pebbled maternal surface. I do not know whether the specimen received was the entire placenta of the surviving offspring, but no more tissue was passed or observed. Published records do not state the length of gestation but at the Duke University Primate Center most animals had a 172 days gestation, with very good breeding dates. Most newborns weighed 110 g; this newborn was 102 g at birth.
 |
This neonate at 10 days of age when it weighed 165 g. |
 |
Neonate showing the characteristic fingers of aye-ayes. |
Mossman (1987) indicated that placentation of this genus is similar to other strepsirhines in being diffuse, villous and epitheliochorial but that little is known about it. There is, however, a very detailed report of two uteri near the end of pregnancy that were well preserved and are superbly illustrated (Hill & Burne, 1922). This report indicates that the placenta extends over both uterine horns, but that the fetus lies in one horn and the uterine body. An extension of chorion proceeded into the cervical canal.
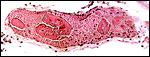
4) General Characterization of the Placenta
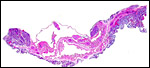
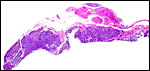
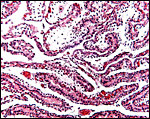
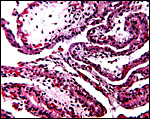
In general, the placenta of aye-ayes is similar to that of other Lemuridae. This is an epitheliochorial placenta with stretches of villous tissue interrupted by membranes with trophoblastic surface that show relatively few villi. The peripheral villi have but few branches. There is a single layer of cuboidal to cylindrical trophoblast. Syncytial cells are not present. The villous connective tissue is sparse and contains very few poorly visualized Hofbauer cells. In the peripheral villi, the fetal capillaries are pressed closely to the under-surface of the trophoblast. There were several brown oval structures beneath the chorion that have been described in some detail by Hill & Burne (1922) and, earlier, in Nycticebus and Galago, by other authors. They have been referred to as "chorionic vesicles" and Hill & Burne, who found 60 of them in each specimen, considered them to represent storage material of uterine secretion. Despite their brown coloration, they are iron-stain negative. It is not entirely clear to me what these structures represent. They appear to have a flattened epithelial surface and contain irregular inclusions. They are not degenerated villi because of their location and not by-products of iron transfer, as in artiodactyla. And they are very irregular and located in a peculiar site that should negate the notion that they are remnants of uterine secretion.
Hill & Burne (1922) described that the villi are finely interdigitated with the endometrial folds. These authors compared the placenta with other prosimians and considered the epitheliochorial, adeciduate placentation to be a primitive form in evolutionary terms.
 |
Fetal surface of fresh Aye-aye placenta. |
 |
Maternal surface of the same placenta. |
This is a typical epitheliochorial placenta as shown in the next few pictures. The only truly unusual features are the chorionic vesicles.
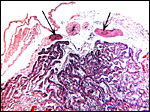
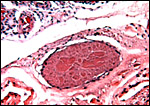
The umbilical cord attached to this placenta was 8.5 cm long, had no spirals, a smooth surface and inserted near the placental margin. Hill & Burne (1922) indicated its length as being 4.3 cm with a 16.6 cm (CR) long fetus. It contained three umbilical blood vessels, one vein and two arteries. Further out in the cord, the arteries divide and five vessels were found. There is also an allantoic duct that is surrounded by dense connective tissue but without smaller blood vessels as those seen in cetacea or artiodactyla. The surface of the cord is composed of a complex multilayered epithelium and, nearer the fetal end, there are numerous superficial inclusions that are composed of keratinizing epithelium - small "pearls".
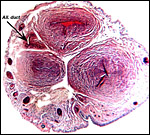 |
Cross section of umbilical cord showing the allantoic duct and the numerous subepithelial squamous inclusions. |
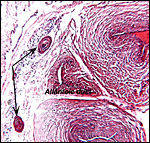 |
Higher magnification with allantoic duct and epithelial inclusions at arrows. |
There are no such studies.
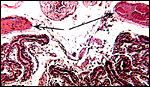
8) Extraplacental membranes
The thin amnion contains no blood vessels and has a single layer of thinned squamous epithelium. There is a potential space between amnion and chorion. The latter contains the large fetal surface blood vessels. There was a large, irregularly shaped (multilobulated) allantoic sac in the uteri examined by Hill & Burne (1922) that was fused to the chorion and contained fetal urine from the cord's allantoic duct. The connective tissue of the allantochorion contains large allantochorial vessels and these extend to some distance but the final pockets of the multichambered allantois contain no capillaries. This unusual feature was already commented upon by Hill & Burne (1922). My specimen does not contain remnants of yolk sac, nor were they found by Hill & Burne (1922).
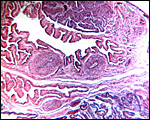 |
Surface with allantochorionic vessels, amnion and allantois. |
There is none.
10) Endometrium
There is probably no decidualization although this specimen did not allow
such judgment. Hill & Burne (1922) found the endometrium to remain
glandular during gestation with signs of secretory activity. The interdigitating
villi of the placenta come into close approximation with the endometrial
surface which is richly vascularized. The uterus is bicornuate and those
authors observed that the cervical canal was not occluded by mucus, as
would be expected in human uteri. Ovaries are contained in a bursa.
11) Various features
None.
12) Endocrinology
I am not aware of any endocrine measurements done on aye-ayes. Eckstein
& Zuckerman (1956) referred to an unpublished thesis by Nayak that
suggested that the testes descend seasonally into the scrotum in this
species, as is described for Loris and Perodicticus.
Females have two abdominal mammae and males have a penile bone.
Aye-ayes have 30 chromosomes with the nucleolus-organizing region confined to the shortest acrocentrics (# 13 & 14) (Rumpler et al., 1989, 1990; Poorman-Allen & Izard, 1990; Tagle et al., 1990; Rakotoarisoa et al., 2000). The Y-chromosome, not depicted here, is a small metacentric chromosome according to the G-banding karyotype published by Poorman-Allen & Izard (1990). Hybrids have not been described. The cytochrome c oxidase gene study of Adkins & Honeycutt (1994) placed the aye-aye as sister taxon to strepsirhini. This was also borne out by the study of the epsilon-globin gene study of Porter et al. (1995), but was not the case from the new study of the cytochrome b gene published by Yoder et al. (1996).
 |
Karyotype of female aye-aye, 2n=30, Giemsa banding arrangement following Poorman-Allen & Izard (1990). |
No publications are known to me.
15) Pathological features
I am unable to find any records of pathological features.
16)
Physiologic data
Oogenesis persists into adulthood (Petterrousseaux & Bourliere, 1965).
17) Other resources
Cells strains of a female specimen are available from CRES
at the Zoological Society of San Diego by contacting Dr. O. Ryder at oryder@ucsd.edu.
18) Other remarks - What additional
Information is needed?
There is great need to better understand the physiology of reproduction,
endocrinology and early implantation in this species.
Acknowledgement
The animal photographs in this chapter come from the Duke University Primate
Center at Durham, NC through the courtesy of David Haring. But, I am most
appreciative to Dean Gibson of the same facility for making the placenta
available to me and for looking after the birth and rearing of this offspring.
References
Adkins, R.M. and Honeycutt, R.L.: Evolution of the primate cytochrome
c oxidase subunit II gene. J. Mol. Evol. 38:215-231, 1994.
Antonius, E.: Lexikon ausgerotteter Vögel und Säugetiere. NTV Wissenschaft, Germany 2003.
Eckstein, P. and Zuckerman, S.: Morphology of the reproductive tract. Pp. 43-155, in Marshall's Physiology of Reproduction., 3rd ed., A.S. Parkes, ed. Vol. I. Longmans, Green & Co. London, 1956.
Feistner, A.T. and Ashbourne, C.J.: Infant development in a captive-bred aye-aye (Daubentonia madagascariensis) over the first year of life. Folia Primatol. 62:74-92, 1994.
Gotch, A.F.: Mammals - Their Latin Names Explained. Blandford Press, Poole, Dorset, 1979.
Groves, C.: Primate Taxonomy. Smithsonian Institution, Washington, D.C 2001.
Hill, J.P. and Burne, R.H.: The foetal membranes and placentation of Chiromys madagascariensis. Proc. Zool. Soc. London 1922. Pp.1145-1165, 1922. [Note: this paper is followed by a brief account of the external features of the fetuses in the specimens described. It is written by R.I. Pocock and five pages long].
Jolly, A.: Madagascar's Lemurs. On the Edge of Survival. National Geographics 174:134-160, 1988.
Jones, M.L.: Successes and failures of captive breeding. Chapter 18 pp 249-260, in Primates - The Road to Self-sustaining Populations. K. Benirschke, ed. Springer-Verlag, N.Y. 1986.
Mossman, H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Nowak, R.M.: Walker's Mammals of the World. 6th ed. The Johns Hopkins Press, Baltimore, 1999.
Pastorini, J., Forstner, M.R. and Martin, R.D.: Phylogenetic relationships among Lemuridae (Primates): evidence from mtDNA. J. Hum. Evol. 43:463-478, 2002.
Petterrousseaux, A. and Bourliere, F.: Persistence of ovogenesis phenomena in the adult form of Daubentonia madagascariensis (Prosimii, Lemuriformes). Folia Primatol. 3:241-244, 1965.
Pollock, J.I.: The management of Prosimians in captivity for conservation and research. Chapter 20 pp. 269-288, in Primates - The Road to Self-sustaining Populations. K. Benirschke, ed. Springer-Verlag, N.Y. 1986.
Poorman-Allen, P. and Izard, M.K.: Chromosome banding patterns of the Aye-aye, Daubentonia madagascariensis (Primates, Daubentoniidae) Intern. J. Primatol. 11:401-409, 1990.
Porter, C.A., Sampaio, I., Schneider, H., Schneider, M.P., Czelusniak, J. and Goodman, M.: Evidence on primate phylogeny from epsilon-globin gene sequences and flanking regions. J. Mol. Evol. 40:30-55, 1995.
Rakotoarisoa, G., Hirai, Y., Go, Y., Kawamoto, Y., Shima, T., Koyama, N., Randrianjafy, A., Mora, R. and Hirai, H.: Chromosomal localization of 18S rDNA and telomere sequence in the aye-aye, Daubentonia madagascariensis. Genes Genet. Syst. 75:299-303, 2000.
Rumpler, Y., Warter, S., Petter, J.J., Albignac, R. and Dutrillaux, B.: Chromosomal evolution of Malagasy lemurs. XI. Phylogenetic position of Daubentonia madagascariensis. Fol. Primatol. 50:124-129, 1988.
Rumpler, Y., Warter, S., Ishak, B. and Dutrillaux, B.: Chromosomal evolution in prosimians. Human Evol. 4:157-170, 1989.
Tagle, D.A., Goodman, M. and Miller, D.A.: Characterization of chromosomes and localization of the rDNA locus in the aye-aye (Daubentonia madagascariensis). Cytogenet. Cell Genet. 54:43-46, 1990.
Tattersall, I. The Primates of Madagascar. Columbia University Press, New York, 1982.
Tattersall, I.: Systematics of the Malagasy strepsirhine primates. Pp. 43-72, in Comparative Primate Biology Vol. 1. Alan R. Liss Inc. 1986.
Yoder,
A.D., Vilgalys, R. and Ruvolo, M.: Molecular evolutionary dynamics of
cytochrome b in strepsirrhine primates: the phylogenetic significance
of third-position transversions. Mol. Biol. Evol. 13:1339-1350, 1996.