| |
10)
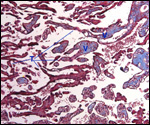
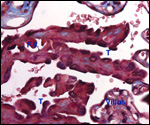
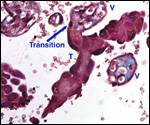
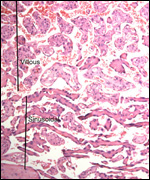
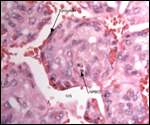
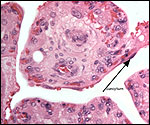
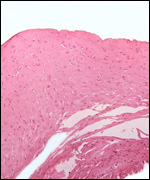
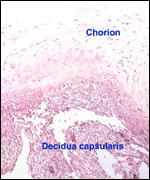
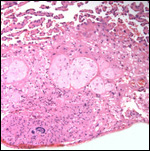
Endometrium
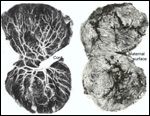
There is little decidua shed with the delivered placenta. Moreover, Walls
(1939) found virtually no decidua vera, and only very little deciduas capsularis
in his first trimester specimen. Little decidual tissue, intermixed with
some trophoblast, is found in the delivered placenta.
11)
Various features
No other significant observations were made.
12) Endocrinology
Ovarian cycles and a pregnancy were examined by fecal steroid analysis
only once (Patzl, et al., 1998). These investigators found the onset of
ovarian cyclic activity after pregnancy to occur within 4-11 weeks. The
pregnancy lasted 184 days. The average length of ovarian cycles was 51
days. Progestogens rose above the cyclic luteal activity in the second
half of pregnancy and were 20 times higher during the week before parturition.
Estrogens rose significantly in the last third of gestation and, especially,
shortly before birth.
A detailed hormonal study of cycles in six animals and study of pregnancy diagnosis comes from the doctoral work of Nicole Schauerte (2005). Cycles were not seasonal and polyestrous with slight vaginal bleeding occurring in the pro-estrus. Ultrasonic determination of pregnancy was also documented.
Additional observations on the endometrium by Becher (1931) are useful.
In his large study of pregnant and nonpregnant uteri of four-toed tamanduas,
Becher described the cyclical change of the endometrium. It had many similarities
with human endometrium, and periods of estrus were described with marked
secretion of mucus from the vagina. In the later stages of the endometrial
cycle, the photographs suggest that there is a decidua-like change of
the endometrial stroma. Much extravasation was reported in later stages
of the cycle and in early pregnancy, Becher described a thick decidua.
He also correlated the endometrial changes with the state of ovarian activity
and described the appearance of the corpus luteum.
13) Genetics
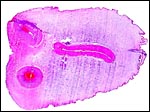
Giant anteaters have 60 chromosomes (Hsu, 1965). We can confirm this from
our own study of four animals. No other chromosomal studies have been
published. Garcia et al. (2005) studied and characterized six microsatellite markers that may become useful for studies of population and paternity.
 |
Karyotype
of male giant anteater from CRES. |
14) Immunology
There are no published reports.
15)
Pathological features
A small number of giant anteaters have been autopsied and only a few specific
lesions were identified. Baskin et al. (1977) found infection with Yersinia
pseudotuberculosis, presumably from contamination of feed by rats
or pigeons. Diniz et al. (1995) identified digestive disorders as most
prominent features, with many other systemic diseases being less common
(infection, nutritional, respiratory, etc.). Parasites of one kind or
another were found in 48% of fecal samples. Griner (1983) listed trauma
as a major problem in captivity (tapir-induced neonatal mortality), and
arteriosclerosis.
RNA viruses (Picobirnavirus) were found in the fecal study by Haga et
al. (1999). Labruna et al. (2002) found numerous ticks (Amblyomma sp. etc.) on these animals as well as many other species from Parana and Mato Grosso. Freitas et al. (2006) recovered Eimeria species in giant anteaters.
Coke et al. (2002) documented gastric perforation (Entameba and Acanthamoeba spp.) in an animal with peritonitis (after perforation) and cardiac myopathy (dilatation).
16)
Physiologic data
There are no reports to my knowledge.
17)
Other resources
Fibroblast cell strains of four animals are available from the "Frozen
Zoo" at CRES
of the San Diego Zoo by contacting Dr. Oliver Ryder at oryder@ucsd.edu.
18)
Additional remarks and needs for future studies
The contribution by Becher (1931) on Tamandua tetradactyla is important
in this context. In the first place, Becher had an unusually large material
of pregnant and nonpregnant uteri available. But, more importantly, he
described the material as having been well fixed for histologic studies.
In addition, he had several stages of fetal/placental development available
that provide more insight than the study of one particular stage. He pointed
out that this is perhaps the reason why his findings differ in many ways
from the interpretation of Wislocki's single specimen of a two-toed anteater
(1928).
I will summarize some salient conclusions from this related species of
anteater that was studied by Becher. These remarks are probably equally
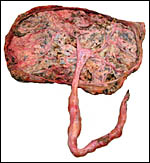
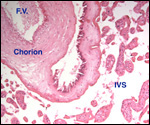
applicable to the placenta of the giant anteater. The placenta was always
implanted at the fundus and, with advancing gestation, the uterine wall
became very thin in the region of nidation. In the fundus, a vascular
network developed in the musculature that was visible externally. Becher
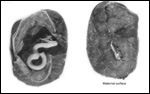
spoke of a "corpus cavernosum". The maternal surface of the
placenta had a finely lobulated appearance. No yolk sac and no allantois
were observed, even in the earliest stages of pregnancy (that is quite
different from what Enders described for the armadillo). The umbilical
cord inserted centrally and the lengths of some cords were given for immature
stages. Becher and other observers reported that the periphery of the
placental disk has a trough. Perhaps this led to the suggestion that it
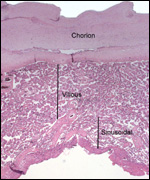
has a bell shape. The study presented an interesting flat section through
the organ. This displayed the "mesodermal villi" (his choice
of words) to be connected to one another by the solid trophoblastic trabeculae.
These, Becher found (in contrast to Wislocki) are composed of large cytotrophoblast
and contain no connective tissue. He found these sheets of trabeculae
to become hyalinized toward term and interpreted them not to become zones
of possible future expansion of the villous structures. Their plump cells
have no microvillous surface. Most interesting to me is his observation
that the placenta shed in the "muscular" (!) cavernous zone
(not in the decidua).
There is much work to be done on the nature of this peripheral zone of
the xenarthran placentas. Most important will be the identification (maternal
vs. fetal) of the individual trabecular elements by genetic means. Electronmicroscopy
will be mandatory also to better understand the contribution of the trabeculae
to the placental development and its function.
Acknowledgement
Most animal photographs of this book come from the Zoological Society
of San Diego. I appreciate their help and that of the pathologists at
the San Diego Zoo.
References
Baskin, G.B., Montali, R.J., Bush, M., Quan, T.J. and Smith, E.: Yersiniosis
in captive exotic mammals. J. Amer. Vet. Med. Assoc. 171:908-912, 1977.
Becher, H.: Placenta und Uterusschleimhaut von Tamandua tetradactyla (Myrmecophaga).
Gegenbauers Morphol. Jahrb. 67:381-458, 1931.
Cell
strains from CRES at: www.sandiegozoo.org
Coke, R.L., Carpenter, J.W., Aboellail, T., Armbrust, L. And Isaza, R.: Dilated cardiomyopathy and amebic gastritis in a giant anteater (Myrmecophaga tridactyla). J. Zoo Wildl. Med. 33:272-279, 2002.
Diniz,
L.S., Costa, E.O. and Oliveira, P.M.: Clinical disorders observed in anteaters
(Myrmecophagidae, Edentata) in captivity. Vet. Res. Commun. 19:409-415,
1995.
Enders,
A.C.: Development and structure of the villous haemochorial placenta of
the nine-banded armadillo (Dasypus novemcinctus). J. Anat. 94:34-45,
1960.
Freitas, F.L., Almeida Kde, S., Zanetti, A.S., do Nascimento, A.A., Machado, C.L. and Machado, R.Z.: Species of the genus Eimeria(Apicomplexa: Eimeriidae) in giant anteaters (Myrmecophaga tridactyla Linnaeus, 1758) in captivity. Rev. Bras. Parasitol. Vet. 15:29-32, 2006. (in Portuguese).
Garcia, J.E., Vilas Boas, L.A., Lemos, M.V.F., de Macedo Lemos, E.G. and Contel, E.P.B.: Identification of microsatellite DNA markers for the giant anteater Myrmecophaga tridactyla. J. Hered. 96:600-6002, 2005.
Griner,
L.A.: Pathology of Zoo Animals. Zoological Society of San Diego, San Diego,
California, 1983.
Haga,
I.R., Martin, S.S., Hosomi, S.T., Vincentini, F., Tanaka, H. and Gatti,
M.S.: Identification of a bisegmented double-stranded RNA virus (Picobirnavirus)
in faces of giant anteaters (Myrmecophaga tridactyla). Vet. J.
158:234-236, 1999.
Hsu,
T.C.: Chromosomes of two species of anteaters. Mammal. Chromosome Newsletter
15:108-109, 1965.
Korniljewa,
I.A. and Roshdestwenskaja, I.D.: Zur Zucht des grossen Ameisenbären,
Myrmecophaga tridactyla L., im Leningrader Zoopark. Zool. Garten
45:377-384, 1975.
Labruna, M.B., de Paula, C.D., Lima, T.F. and Sana, D.A.: Ticks (Acari:Ixodidae) on wild animals from the Porto-Primavera Hydroelectric power station area, Brazil. Mem. Inst. Oswaldo Cruz 97:1133-1136, Epub 2003 Jan. 20.
Montgomery,
G.G., ed.: The Evolution and Ecology of Armadillos, Sloths, and Vermilinguas.
Smithsonian Institution, Washington, 1985.
Mossman,
H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Nowak,
R.M.: Walker's Mammals of the World. 6th ed. The Johns Hopkins University
Press, Baltimore, 1999.
Patzl,
M., Schwarzenberger, F., Osmann, C., Bamberg, E. and Bargmann, W.: Monitoring
ovarian cycle and pregnancy in a giant anteater (Myrmecophaga tridactyla)
by faecal progestagen and oestrogen analysis. Anim. Reprod. Sci. 53:209-219,
1998.
Puschmann,
W.: Zootierhaltung. Vol. 2, Säugetiere. VEB Deutscher Landwirtschaftsverlag
Berlin, 1989.
Schauerte, N.: Untersuchungen zur Zyklus- und Graviditätsdiagnostik beim Grossen Ameisenbären (Mymecophaga tridactyla). (Giessener Elektronische Bibliothek:URN:urn:nbn:de:hebis:26-opus-28121). This is available from: http://geb.uni-giessen.de/geb/volltexte/2006/2812/
Walls,
E.W.: Myrmecophaga jubata: An embryo with placenta. J. Anat. 73:311-317,
1939. [Note: M. jubata is synonymous with M. tridactyla
- see Wilson & Reeder, 1992].
Wilson
D.E. and Reeder, Eds.: Mammal Species of the World. Second Ed. Smithsonian
Inst. Press, Washington, 1992.
Wislocki,
G.B.: On the placentation of the two-toed anteater (Cyclopes didactylus).
Anat. Rec. 39:69-83, 1928.
|