| (Clicking
on the thumbnail images will launch a new window and a larger version
of the thumbnail.) |
| Last updated: Dec 30, 2005. |
Bubalus (Anoa) depressicornis
Order: Artiodactyla
Family: Bovidae
1) General zoological data
The subgenus Anoa contains two species according to Groves (1969), the mountain anoa ( B. quarlesi ) and the species described here, the lowland anoa. It is severely endangered and is not commonly bred successfully in zoological parks. Anoas are the smallest of surviving forest buffalos from Asia ( Sulawesi - Celebes Islands ) with weights of 150-300 kg (Wünschmann, 1968; Nowak, 1991). It is most closely related to the water buffalos. The phylogenetic derivation has been well examined from a variety of findings (skeletal, soft tissue, chromosomes) by Groves (1981). He and Pilgrim (1947) derive Bubalus from the ancestral Proamphibos . Anoa is the Sulawesi word for "buffalo", as this animal is often referred to as a ‘miniature water buffalo'.
 |
Lowland anoa at San Diego Zoo. |
2) General gestational data
The gestation of anoas is described as being 275-315 days long with generally single offspring and longevity of up to 28 years; Jones (1993) even lists 36 years as the maximum life span.
3) Implantation
Little information is available on early stages of anoa placentation, but Austin (1994) depicts a 16 cell egg of a swamp buffalo ( Bubalus bubalis ) that was 150 µm in diameter. Further stages are not described.
4) General Characterization of the Placenta
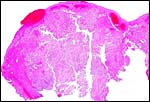
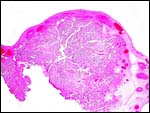
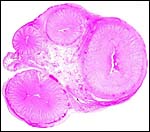
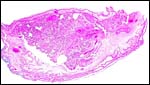
I have not been able to find any description of the anoa placenta, but Mossman (1987) refers to various contributions on the placenta of water buffalo (e.g. el-Naggar & Abdel-Raouf, 1971). The anoa placenta may be quite similar to that of the water buffalo, although it is of course much smaller; moreover, hybridization has not been successful. The placenta of this animal was received after a Cesarean section had been done because of dystocia during labor. The presumably female neonate (see section on pathology, however) was deeply black, weighed 6,425 g (53 cm CR), and the placenta weighed 375 g. The placenta that was removed at section measured 60 cm in greatest length and had only 25 cotyledons. Regretfully, it was incomplete and three days later retained placental tissue had to be removed. Thus, the complete size of an anoa placenta remains unknown. The largest cotyledon measured 7 cm in greatest diameter and was 2 cm thick. The maternal cotyledonary surfaces were very irregular and much different from those seen in most Artiodactyla; the fetal surface was ‘flat' in Mossman's (1987) terminology. Regrettably, because of the post mortem interval some bacterial growth had taken place on portions of the placenta which were moderately autolyzed. The better preserved regions are here depicted.
 |
Macroscopic appearance of lowland anoa placenta, disrupted by the removal after Cesarean section. |
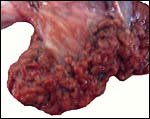 |
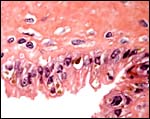
Surface appearance of the largest cotyledon. Note the irregular nature of the maternal surface. |
5) Details of fetal/maternal barrier
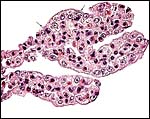
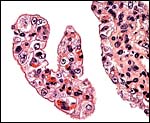
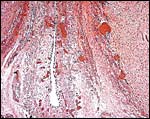
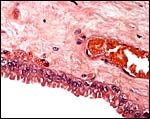
The trophoblastic epithelium is cuboidal and rarely binucleate; but, as other bovids, the anoa has some binucleate trophoblastic cells on the villous surfaces. Wimsatt (1951) described their morphology in sheep and cattle in some detail. He concluded that their binucleate status arises by mitosis but that the cells then become post-mitotic. In his reexamination of binucleate trophoblast, Wooding (1982) affirmed their production of placental lactogen and showed in several Artiodactyla that the cells fuse with endometrial epithelial cells, to deliver their secretory product into the maternal circulation while maintaining an epithelio-chorial placentation.
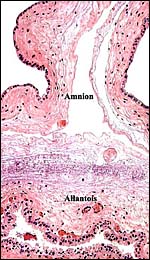
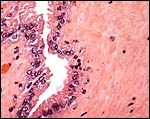
In some contrast to other bovid placentas I have been able to study, this villous tissue had more finely divided ramifications at the periphery; indeed, some peripheral villi had the appearance of being largely composed of trophoblast with tiny fetal capillaries in the diminutive amount of central connective tissue. Also, as in many other ruminants, the trophoblast beneath the chorionic plate – and only here – had some yellow apparently crystalline cytoplasmic inclusions. They are depicted below and may represent similes of the ‘hematophagous organ'.
6) Umbilical cord
The cord of this disrupted placenta measured only 5 cm in length, had no twists, and it contained two arteries and two veins, as well as an allantoic duct. No additional cord was present on the calf. The surface was smooth. The microscopic aspects of the umbilical cord show an unusually large number of small blood vessels, many of which are found under the surface epithelium. Some of these vessels can be seen to emanate from the thick muscular blood vessels.
7) Uteroplacental circulation
There are no data.
8) Extraplacental membranes
There is a large allantoic sac. It is lined by cylindrical epithelium that, despite the autolysis, appears to have a microvillous surface. It is also richly vascularized for water retrieval. The amnionic epithelium is flat, squamous, has no ‘pearls' and the connective tissue is avascular.
9) Trophoblast external to barrier
None has been reported and it is unlikely to be present.
10) Endometrium
Endometrial changes of pregnancy have only been reported for water buffalo (Bhavsar et al., 1974), not the anoa.
11) Various features
No decidua is formed in anoa gestations. Abdel-Raouf & el-Naggar (1969) have reported on the biometry of gravid water buffalo cows, but not on anoas.
12) Endocrinology
The length of the reproductive cycle in the water buffalo is around 20-21 days. Hafez & Attar (1955) measured estrogens in water buffalo but not the anoa.
13) Genetics
Anoas have 48 chromosomes, the first row of six pairs of the karyotype displayed below being metacentric or submetacentric elements. The first authors to describe the chromosome number of anoas were Nelson-Rees et al. (1969); then Koulisher (1971) and Low & Benirschke, (1973) described even initial banding patter in anoa chromosomes. Koulisher et al. (1972) have published the karyotype of the mountain anoa; it has a only 2n=46, with 7 pairs of metacentrics. Thus, the NF is like that of cattle. Other studies at the Ragunan zoo (web site) confirm this finding. A related species, the tamaraw ( Anoa mindorensis ) was studied by Fischer & Höhn (1973). It had 2n=46 and a morphology very similar to that of the mountain anoa.
From the sequence of the cytochrome b gene, Tanaka et al. (1996) deduced that mountain and lowland anoas are definitely different species and placed the divergence time at 2 million years. Moreover, the tamaraw from the Philippines was classified as a subgenus of Bubalus , but not Anoa . Schreiber et al. (1999) further examined the cytochrome b gene polymorphism of anoas and suggested that it would be a useful tool for the genetic management of captive anoas. Sena et al. (2003) studied MHC alleles in water buffalo, anoa and Syncerus , finding significant polymorphisms.
While mating between cattle and water buffalo has often been reported, no hybrids have been produced (Gray, 1972).
 |
Karyotype of male lowland anoa from the San Diego Zoo, CRES. |
14) Immunology
I am not aware of any data.
15) Pathological features
Griner (1983) recorded 15 autopsies done in anoas at San Diego Zoo and found trauma as a cause of death in three, ‘shock' in three, and ‘bloat' in one. Of nine births, four were stillbirths . Flach et al. (2002) observed that gamma herpesvirus DNA was detected in adult and newborn lowland anoas at the Whipsnade Wild Animal Park, UK. In a survey of mammals in the Barcelona zoo, Gomez et al. (2000) identified Cryptosporidium sp.
The animal here discussed was stillborn after dystocia developed during labor that necessitated a Cesarean section. It was anomalous, however: It had a penis, no testes, one uterine horn only and an ovary on that side. Cytogenetic studies are underway.
16) Physiologic data
Hematological data are available on water buffalo, but not on the anoa (Hafez & Anwar, 1954). Abdel-Raouf et al. (1974) have described the structure of the fetal testis in water buffalo.
17) Other resources
Cell strains are available from CRES at the San Diego Zoological Society, including from this abnormal neonate.
18) What additional information is needed?
Obviously, many more placentas need to be studied before the normal structure shown here can resolve the many questions that remain.
Acknowledgement
The animal photograph in this chapter comes from the Zoological Society of San Diego. I appreciate also very much the help of the pathologists at the San Diego Zoo.
ReferencesAbdel-Raouf, M. and el-Naggar, M.A.: The biometry of the gravid uterus in Egyptian buffalo cows. Zentralbl. Veterinaermed. A. 16:838-854, 1969.
Abdel-Raouf, M., el-Naggar, M.A. and el-Bab, M.R.: The development of the fetal testis in the buffalo. Z. Anat. Entwicklungsgesch. 144:227-236, 1974.
Austin, C.R.: Preimplantation development. Chapter 2 in, Marshall's Physiology of Reproduction, Fourth edition, Vol. 3, Part 1, G.E. Lamming, ed., Chapman & Hall, London, 1994.
Bhavsar, B.K., Pattabiraman, S.R. and Venkataswami, V.: Endometrial changes during early pregnancy of Bubalus bubalis . Indian Vet. J. 51:175-176, 1974.
el-Naggar, M.A. and Abdel-Raouf, M.: The foetal membranes and fluids in the Egyptian buffalo. Zentralbl. Veterinaermed. A 18:108-123, 1971.
Fischer, H. and Höhn, H.: Der Karyotyp eine weiblichen Tamarau (Anoa mindorensis). Giessener Beitr. Erbpath. Zuchthyg. 6:173-177, 1973.
Flach, E.J., Reid, H., Pow, I. and Klemt, A.: Gamma herpesvirus carrier status of captive artiodactyls. Res. Vet. Sci. 73:93-99, 2002.
Gomez, M.S., Torres, J., Gracenea, M., Fernandez-Moran, J. and Gonzalez-Moreno, O.: Further report on Crytosporidium in Barcelona zoo mammals. Parasitol. Res. 86:318-323, 2000.
Gray, A.P.: Mammalian Hybrids. A Check-list with Bibliography. 2 nd edition.
Commonwealth Agricultural Bureaux Farnham Royal, Slough , England , 1972.
Griner, L.A. : Pathology of Zoo Animals. Zoological Society of San Diego , San Diego , California , 1983.
Groves , C.P.: Systematics of the anoa (Mammalia, Bovidae). Beaufortia 17:1-12, 1969.
Groves , C.P.: Systematic relationships in the Bovini (Artiodactyla, Bovidae). Z. zool. Systematik Evolutionsforsch. 19:264-278, 1981.
Hafez, E.S. and Anwar, A.: Normal haematological values in the buffalo. Nature 174:611-612, 1954.
Hafez, E.S. and Attar, T.: Urinary oestrogens in the buffalo as measured chemically. Nature 176:796, 1955.
Jones, M.L.: Longevity of ungulates in captivity. Intern. Zoo Yearbk. 32:159-169, 1993.
Koulisher, L.: Clonal cellular evolution and speciation in mammals: Cytogenetic analogies. Boll. Di Zool. (Ital.) 38:311-316, 1971.
Koulisher, L., Tyskens, J. and Mortelmans, J.: Mammalian cytogenetics. VI. The chromosomes of a male specimen of Anoa depressicornis quarlesi . Acta Zool. Pathol. Antverp. 56:21-24, 1972.
Low, R.J. and Benirschke, K.: The chromosome complement and banding pattern of the lowland Anoa, Anoa depressicornis depressicornis . Chrom. Inform. Serv. 15:23-25, 1973
Mossman, H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Nelson-Rees, W.A., Scott, C.D., Kniazeff, A.J. and Malley, R.L.: Are karyotypes of male and female dwarf buffalo, Anoa depressicornis, superficially alike? In Vitro 4:164, 1969.
Nowak, R.M., ed.: Walker 's Mammals of the World . 5th Edition. The Johns Hopkins University Press, Baltimore and London . Vol.1, p. 629, 1991.
Pilgrim, G.E.: The evolution of the Buffaloes, Oxen, Sheep, and Goats. Linn. Soc. Zool. 41:272-286, 1947.
Schreiber, A., Seibold, I. , Nötzold, G. and Wink, M.: Cytochrome b gene haplotypes characterize chromosomal lineages of anoa, the Sulawesi dwarf buffalo (Bovidae: Bubalus sp.). J. Hered. 90:165-176, 1999.
Sena, L., Schneider, M.P., Brenig, B., Honeycutt, R.L., Womack, J.E. and Skow, L.C.: Polymorphisms in MHC-DRA and –DRB alleles of water buffalo ( Bubalus bubalis ) reveal different features from cattle DR alleles. Anim. Genet. 34:1-10, 2003.
Tanaka, K., Solis, C.D., Masangkav, J.S., Maeda, K., Kawamoto, Y. and Namikawa, T.: Phylogenetic relationship among all living species of the genus Bubalus based on DNA sequences of the cytochrome b gene. Biochem. Genet. 34:443-452, 1996.
Wimsatt, W.A.: Observations on the morphogenesis, cytochemistry, and significance of the binucleate giant cells of the placenta of ruminants. Amer. J. Anat. 89:233-282, 1951.
Wooding, F.B.P.: The role of the binucleate cell in ruminant placental structure. J. Reprod. Fertil. Suppl. 31:31-39, 1982.
Wünschmann, A.: Chapter 13: Die Rinder. In, Grzimeks's Tierleben.Vol. 13, Kindler Verlag, Zürich, Switzerland, 1968.