| March 21, 2007. |
Capra ibex ibex
East Caucasian Tur
Capra cylindricornis
Turkmenian Markhor
Capra falconeri
Order: Artiodactyla
Family: Bovidae
1) General Zoological Data
There are eight recognized ("good") species of goats, and many subspecies exist as well (Nowak, 1999). Material adequate for study was available from three of these species, and the findings of their placental morphology were, in general, quite similar. Nevertheless, minor differences were identified in their placentas and membranes. For that reason these are so indicated separately below. The reader should also refer to my chapters on the Sheep, Cretan Goat, and on the Nubian Ibex. Nievergelt (1966) has published a detailed ecological study of the Alpine ibex.
The origin of the domestic goat, perhaps the first ruminant animal to be domesticated by humans, and the sheep has been of interest for years, and it is still in dispute. Numerous osteological and genetic studies have attempted to clarify this puzzle. Harris (1962) concluded that it was the Bezoar goat (with at least five subspecies) that was the ancestral matriarchal form. This was further supported by evidence from an mtDNA study by Takada et al. (1997) on a relatively small sample of goats. More recently, satellite DNA studies (Curtain et al., 1973), and mtDNA cytochrome b sequences were used to arrive at more modern views of these ancestral relations (Hassanin et al. 1998). These studies, while also still not conclusive, relate C. ibex and C. hircus very closely. Mannen et al. (2001) sequenced the entire mtDNA displacement loop region and also the cytochrome b gene of several Laotian strains of goat and of markhor, and considered the markhor to be very closely related as well. The Tur is presumably more distant. More recently, Pidancier et al. (2006) studied other regions of the mtDNA and the DNA of the Y-chromosome of a variety of goat species and found two possible ancestral trees, without coming to a complete resolution of the discrepancies.
The Alpine ibex was once widely distributed over the European Alps but it is now restricted to a few protected areas (Harris, 1962). Careful measures of protection and some reintroductions of a dwindling number of animals have lead to a remarkable recovery of this subspecies (Nowak, 1999). Its phenotype, with the flat ridges across the broad anterior portions of the flat horns and the small beard, is characteristic. Longevity in captivity was given by Jones (1993) as being 18 years and 9 months. Age can be estimated by the annually increasing number of rings on the horns. Gestation lasts 150-180 days, with usually single births of 2-4 kg (Nowak, 1999). Twins occur only rarely. Puschmann (1984) gives the length of gestation as 155-170 days.
The East Caucasian Tur has more cylindrical horns with prominent ridges and they are sloped to the back. The males also have beards. Like the somewhat different West Caucasian Tur, the bodies are massive. Their gestation lasts 150-160 days and single births (3-4 kg) are the norm. Longevity in the wild is short (10 years), while in captivity they may live to 15 years (Jones, 1993).
The Turkmenian Markhor has very characteristic screw-like spiraled horns in both sexes, although those of males are much more impressive. Gestation lasts 135-170 days according to Nowak (1999), and 140-150 days according to Puschmann (1984). Twins are common and the neonatal weight is 1.5-2.5 kg. The animal is endangered. Longevity is 14 years.
 |
Male Alpine Ibex at San Diego Zoo. |
 |
East Caucasian Tur at San Diego Zoo. |
 |
Markhor at San Diego Wild Animal Park. |
 |
Group of Markhor at San Diego Zoo. |
Alpine Ibex: Gestational length is 150-180 days, usually singletons are born weighing 2-4 kg.
East Caucasian Tur: Gestational length is 150-160 days, singletons, rarely twins. Neonatal weight is 3-4 kg.
Turkmenian Markhor: Gestational length is 135-170 days, singletons are born but twins occur often, weighing 1.5 - 2.5 kg.
3)
Implantation
No studies of implantational stages in these three species are known to
me, and none may have been published. Considering the relationship to
sheep, however, one may expect that the goat's implantation is generally
similar to that of the well-studied sheep and I refer the reader to the
superb description of this feature written by Amoroso (1961). Implantation
occurs over the caruncles of the endometrium, areas of generally fibrous
endometrium that are covered by tall epithelium but contain no glands.
Amoroso considered it as settled that the surface epithelium that covers
the caruncles (he refers to these as "burrs") is destroyed in
placentation, a feature that has been hotly debated in the past. When
the villi form, they interdigitate with clefts in the caruncles and are
separated one from another by maternal fibrous septa (Amoroso refers to
these as "intercrypt columns") . The edges of the cotyledons
then "curl inwards", so as to "hold the cotyledon in place".
It is essential to understand this aspect of cotyledonary development
as only thus can it be understood that the convex outsides of the cotyledons
are covered by maternal endometrial surface epithelium. I recommend highly
that this chapter by Amoroso be read and its excellent illustrations understood.
Of interest in the present chapter is that the nature of the cotyledons
of ruminants differs so much, features that have led Mossman (1987) to
speak of "flat cotyledons" (deer), "concave cotyledons"
(sheep, goat), and "convex cotyledons" (cow). Their gross appearances
are otherwise roughly similar, but the student can easily get lost when
expecting one to have the same microscopic structure as another.
4) General Characterization of the Placenta
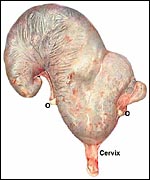
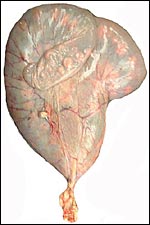
I have had one pregnant uterus available from an Alpine ibex. It
came from a female who was euthanized because of major joint injuries.
She was pregnant with an immature singleton in the left uterine horn.
The placenta extended into the right uterine horn with two vessels from
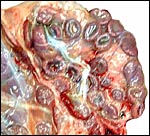
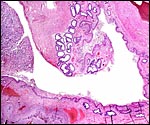
the umbilical cord accompanying this portion of the placenta. This polycotyledonary
placenta has its concave cotyledons appear as though they grow on a stalk.
That would be much more typical of the arrangement found in cows and I
presume therefore that the appearance in this specimen is due to the greater
contraction (during fixation in Bouin's solution) of the myometrium than
of the villous structures. Other specimens did not show this striking
stalk-like attachment. The pregnancy depicted here was approximately 2/3
way through gestation with the male fetus weighing 1,210 g and measuring
32 cm in length. There were 50 cotyledons in each horn. The entire uterus
with contents weighed 3,050 g.
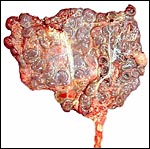
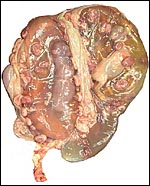
Also available was the uterus of a Turkmenian Markhor (Capra
falconeri). The female had died from aggression when placed with another
animal and was pregnant with immature twins, a male and a female fetus.
The uterus weighed, unopened, 2,400 g (1,200 g after removal of fluids
and fetuses), and the immature fetuses weighed 200 g each and measured
18 cm in crown-rump length. Both uterine horns were occupied and each
placenta had 40 cotyledons arranged in four rows. There were no vascular
anastomoses between the fetal circulations. The cotyledons were typically
concave and measured up to 3 cm in diameter. The remainder of this placenta
was essentially identical to that of the ibex.
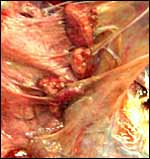
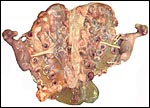
In addition, the very similar, delivered, term placenta of an East
Caucasian Tur (Capra cylindricornis) was available for study.
It weighed 400 g and had only 38 cotyledons (from 2 to 4 cm in size).
The autolytic degeneration, however, made histologic study unsuitable.
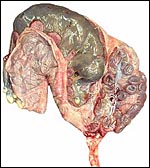
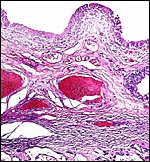
The villi of all three species are slender and interdigitate with the, relatively even more slender, maternal fibrous septa whose covers consist of flat, deeply-stained epithelium. There is no trophoblastic invasion of the endometrium to speak of (perhaps minimal endometrial destruction occurs at the margins of cotyledons), and binucleate cells are sparse and scattered over the otherwise cuboidal trophoblastic surface. Foci of calcification occur normally in the debris between villi and septa, and especially in the tips of occasional maternal septa. The trophoblast beneath the chorionic surface is taller, cylindrical and heavily pigmented with yellow flakes and granules. Much less pigment is present between the cotyledons.
This pigment has been of interest to me. It has usually been assumed to derive from maternal hemorrhages, and this region of ruminant placentas has been referred to as the "hematophagous region". It has been presumed that this area serves the fetus to obtain much iron from the dam. Iron stains have been consistently negative, however, and the "Gmelin" reaction for hematoidin (bilirubin) has failed to disclose that substance as representing the hemoglobin-derived pigment (see the chapter on urial sheep). Furthermore, the lack of solubility in organic solvents (alcohol, xylene, chloroform) does not match the expected quality of hematoidin. On the other hand, it is difficult to imagine that other pigments should exist in this location. To be inclusive, however, I have bleached the slides with peroxide (this is used in order to bleach melanin in histochemical preparations), and the pigmentation was abolished. Next I performed the "Schmorl" stain for melanin and this was focally positive. It was similar to the pigmentation of basal skin strata in control slides, but not quite so dark and uniformly black, as can be seen in the next photograph. While it is difficult to imagine that melanin gets into these specific trophoblastic cells, it is presently the more likely type of pigment, rather than being one of a blood derivative. Alternatively, it may be "ageing pigment", such as found in heart muscle, and occasionally referred to as "promelanin". It is of interest then to recall Amoroso's (1961) statement of the presence of melanoblasts in the sheep endometrium, as had been determined by Grant (1933). Perhaps the same pertains to exotic species and this aspect would be worthy of future study. For this reason then I performed silver stains that normally display melanin pigment, and it was positive also. I conclude, therefore, that this "hematophagous organ" is not likely the site of blood breakdown (and fetal iron absorption) but that it results from the degeneration of endometrial septal tips (observed) with release of their melanin. Perhaps differences in melanin content of ruminant endometria exist to explain the great variability of the amount of pigment found here.
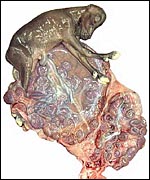
The Alpine ibex cord measured 16 cm and was covered with fine yellow granules. That of the Tur was also 16 cm long. The umbilical cords of the younger Markhor fetuses were 9 cm long. All contained four large blood vessels and a large allantoic duct lined by urothelium. The smaller blood vessels of the cord congregated strikingly around the allantoic duct, more so than is found in other Artiodactyla, and they were often seen to emerge from the larger blood vessels. The cords had no spiraling but numerous plaques of squamous metaplasia were found on the surfaces.
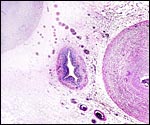 |
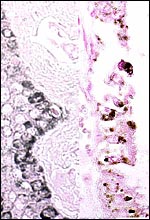
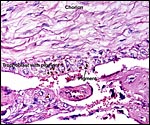
Center of ibex umbilical cord with large (muscular) allantoic duct that is accompanied by numerous blood vessels. |
Amoroso (1961) has shown some of the sheep placental vasculature, and sheep have served as experimental animals to understand gestational problems better for many years. An abundant literature is available that discusses blood flow but it cannot be cited in this brief context. The reader is referred to studies by Barcroft, Barron, Metcalfe, Battaglia, Meschia, and others.
8) Extraplacental membranes
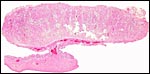
The membranes between the cotyledons are covered with cylindrical trophoblast that is occasionally pigmented. The large allantoic sac lies next (inside) to it and has its usual small caliber vasculature and a cuboidal to cylindrical epithelium that is not pigmented and that has relatively clear cytoplasm. Small amounts of yellow hippomanes were found in the allantoic fluid of the Alpine ibex. The amnion is covered with thin epithelial cells and is avascular. Remnants of yolk sac are not found.
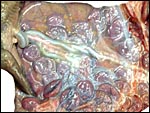
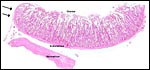
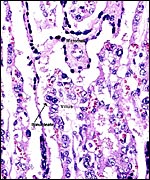
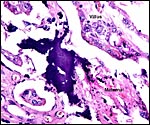
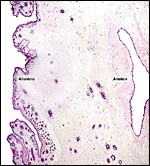
The endometrium has a tall columnar surface epithelium. Beneath this are numerous glands, but very few are found beneath the cotyledons. Here, the endometrium has a fibrous appearance. The surface epithelial sheet of endometrium covers the outside of the cotyledons to their mid to upper portions, as is seen in the low power photograph shown above.
Between the endometrial surface and the intercotyledonary membranous trophoblast is a large amount of scattered debris. It is of uncertain origin. It is not pigmented and has often a granular feature; elsewhere it appears as though being composed of debrided squamous flakes.
There is no convincing evidence for invasion of the maternal tissues.
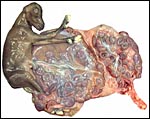
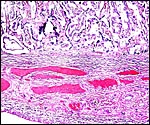 |
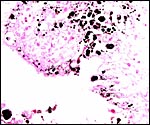
Implanted ibex cotyledon on the fibrous endometrium that lacks glands at this location. |
No decidua in the true sense exists. The endometrial stroma becomes rather more fibrous at the site of implantation than it was in the nonpregnant caruncle.
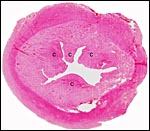 |
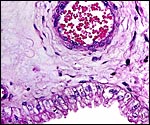
Cross section of pygmy goat uterus (C=caruncles). |
There is no subplacenta.
12)
Endocrinology
The left ovary of the Alpine ibex had a single corpus luteum of pregnancy.
Neonatal organs of other ibex specimens available show no endocrine stimulation
of fetal ovaries or testes.
While no work has been reported on ibex, there is much information of
the placental function in sheep and goat. This is adequately summarized
by Porter et al. (1982). It is generally accepted that the sheep placenta
produces progesterone in later gestation and that the pregnancy becomes
independent of the corpus luteum activity. This does not appear to be
the case in goat placentas. For that reason, it is conjectured, it is
that sheep x goat hybrid pregnancies are compromised. The generation of
gonadotropins from the placenta has not been reported (Courrier, 1945).
The contribution by Porter et al. (1982) also discussed relaxin production
and onset of labor.
13)
Genetics
The Alpine ibex, like most other goat species, and those of markhor and
tur, has 60 chromosomes, all acrocentrics (Schmitt & Ulbrich, 1968).
The structure of ibex chromosomes has been compared with that of domestic
goats by Hauschtek-Jungen & Meili (1967). Only minor differences in
lengths were seen and it was thus not surprising to the authors that hybrids
are fertile. Numerous hybrids with other goat species have been reported
(Gray, 1972) but successful hybridization of ibex with sheep is doubted.
In domestic goats, a number of chromosomal abnormalities, hermaphrodites
and chimeras have been recorded. Such anomalies were especially prevalent
in Saanen dairy goats (Basrur & Coubrough, 1964; Padeh et al., 1965;
Soller et al., 1966). Meiosis was studied by Datta (1970).
Mitochondrial DNA studies are mentioned at the beginning of this chapter.
Most recently, Maudetr et al. (2002) have used microsatellite DNA for
the conservation management of Alpine ibex.
14)
Immunology
Immunoglobulins were studied by Curtain & Fudenberg (1973).
15)
Pathological features
Griner (1983) listed as principal causes of death various types of trauma.
Verminous pneumonia was another major cause of death in all three species,
and trichuriasis was another frequent finding. No proliferative disorders
were seen. Giacometti et al. (2002) described the frequent occurrence
of Mycoplasma conjunctivae-induced keratoconjunctivitis of Alpine
ibex and Chamois. It is fatal in up to 30%. Earlier (1995) they had identified
a high prevalence of antibodies against Chlamydia psittaci, which
is also a serious infection in sheep. It
has now been documented that the mycoplasma that causes the keratoconjunctivitis
in ibex and the chamois is primarily carried in domestic sheep (Janovsky
et al., 2002). Ferroglio et al. (1998) identified Brucella melitensis
infection in Alpine ibex. Several nematode infections were diagnosed by
Zaffaroni et al. (2000).
16)
Physiologic data
The amount of glycosylated hemoglobin in blood (0.425%), and glucose levels
(111 mg/dl) of markhor were determined by Richter (1986).
17)
Other resources
Cell strains of this species and of other goat species are available from
CRES
at the Zoological Society of San Diego by contacting Dr. Oliver Ryder
at oryder@ucsd.edu.
18)
Other remarks - What additional Information is needed?
It would be desirable to have more endocrine studies and early implantational
studies on these species. The placental weight at term is not recorded
for ibex.
Acknowledgement
The animal photographs in this chapter come from the Zoological Society
of San Diego. I appreciate also very much the help of the pathologists
at the San Diego Zoo.
References
Amoroso, E.C.: Placentation. Chapter 15, pp.127-311, In, Marshall's Physiology
of Reproduction, V.II, A.S. Parkes, ed. Second Edition. Little, Brown
& Co. Boston, 1961.
Basrur, P.K. and Coubrough, R.I.: Anatomical and cytological sex of a
Saanen goat. Cytogenetics 3:414-26, 1964.
Courrier, R.: Endocrinologie de la Gestation. Paris, 1945.
Curtain, C.C. and Fudenberg, H.H: The evolution of the immunoglobulin antigens in the Ruminantia. Biochem. Genet. 8:31, 1973.
Curtain, C.C., Pascoe, G. and Hayman, R.: Satellite DNA in the sheep and goat. Biochem. Genet. 10:253-262, 1973.
Datta, M.: Reinvestigation of meiosis in the male goat Capra hircus, Linn., with special reference to chiasma formation in the sex and autosomal bivalents. Cytologia 35:344-353, 970.
Ferroglio, E, Tolari, F., Bollo, E. and Bassano, B.: Isolation of Brucella melitensis from alpine ibex. J. Wildl. Dis. 34:400-402, 1998.
Giacometti, M., Tolari, F., Mannelli, A. and Lanfranchi, P.: Seroepidemiologic investigations in the Alpine ibex (Capra i. ibex) of Piz Albris in the canton of Grigioni (Switzerland). Schweiz. Arch. Tierheilk. 137:537-542, 1995.
Giacometti, M., Janovsky, M., Belloy, L. and Frey, J.: Infectious keratoconjunctivitis of ibex, chamois and other Caprinae. Rev. Sci. Techn. 21:335-345, 2002.
Grant, R.: The pigmentation of the uterine mucosa in the ewe. Vet. J. 89:271, 1933 (quoted by Amoroso).
Gray, A.P.: Mammalian Hybrids. A Check-list with Bibliography. 2nd edition. Commonwealth Agricultural Bureaux Farnham Royal, Slough, England, 1972.
Griner, L.A.: Pathology of Zoo Animals. Zoological Society of San Diego, San Diego, California, 1983.
Harris, D.R.: The distribution and ancestry of the domestic goat. Proc. Linn. Soc. London 173:79-91 1962.
Hassanin,
A., Pasquet, E. and Vigne, J.-D.: Molecular systematics of the subfamily
caprinae (Artiodactyla, Bovinae) as determined from cytochrome b sequences.
J. Mammal. Evol. 5:217-236, 1998.
Janovsky, M., Frey, J., Nicolet, J., Belloy, L. and Giacometti, M.: Mycoplasma
conjunctivitis is maintained in domestic sheep but not in Alpine chamois
in the Swiss Alps. Pp.33-34, In Proceed. Europ. Assoc. Zoo- and Wild.
Veterinarians, 4th scientific meeting, Heidelberg, Germany, May 8-12,
2002.
Hauschteck-Jungen, E. and Meili, R.: Vergleich der Chromosomensätze von Steinwild (Capra ibex) und der Hausziege (Capra hircus). Chromosoma 21:198-210, 1967.
Jones, M.L.: Longevity of ungulates in captivity. Intern. Zoo Yearbk. 32:159-169, 1993.
Mannen, H., Nagata, Y. and Tsuji, S.: Mitochondrial DNA reveal that the domestic goat (Capra hircus) are genetically affected by two subspecies of bezoar (Capra aegagurus) Biochem. Genet. 39:145-154, 201.
Maudetr, C., Miller, C., Bassano, B., Breitenmoser-Wursten, C., Gauthier, D., Obexer-Ruff, G., Michallet, J., Taberlet, P. and Luikart, G.: Microsatellite DNA and recent statistical methods in wildlife conservation management: applications in Alpine ibex [Capra ibex ( ibex)]. Molec. Ecol. 11:421-436, 2002.
Mossman,
H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Nievergelt, B.: Der Alpensteinbock (Capra ibex L.) in seinem Lebensraum.
Paul Parey, Hamburg, 1966.
Nowak, R.M.: Walker's Mammals of the World. 6th ed. The Johns Hopkins Press, Baltimore, 1999.
Padeh, B., Wysoki, M., Ayalon, N. and Soller,M.: An XX/XY hermaphrodite in the goat. Israel J. Med. Sci. 1:1008-1012, 1965.
Pidancier, N., Jordan , S., Luikart, G. and Taberlet, P.: Evoluionary history of the genus Capra (Mammalia, Artiodactyla): Discordance between mitochondrial DNA and Y-chromosome phylogenies. Mol. Phylogen. Evol. 40:739-749, 2006.
Porter, D.G., Heap, R.B. and Flint, A.P.F.: Endocrinology of the placenta and the evolution of viviparity. J. Reprod. Fertil., Suppl. 31:113-138, 1982.
Puschmann, W.: Zootierhaltung. Vol. 2, Säugetiere. VEB Deutscher Landwirtschaftsverlag Berlin, 1989.
Richter, N.A: Percentage of glycosylated hemoglobin and serum concentration of glucose in the blood of Japanese macaques and in three exotic ruminant species. Amer. J. Vet. Res. 47:1783-1784, 1986.
Schmitt, J. and Ulbrich, F.: Die Chromosomen verschiedener Caprini Simpson, 1945. Z. Säugetierk. 33:180-186, 1968.
Soller,M., Wysoki, M. and Padeh, B.: A chromosomal abnormality in phenotypically normal Saanen goats. Cytogenetics 5:88-3, 1966.
Takada, T., Kikkawa, Y., Yonekawa, H., Kakami, S. and Amano, T.: Bezoar (Capra aegagrus) is a matriarchal candidate for ancestor of domestic goat (Capra hircus): evidence from the mitochondrial DNA diversity. Biochem. Genet. 35:315-326 197
Zaffaroni, E., Teresa Manfredi, M., Citterio, C., Saa, M., Picclo, G. and Lanfranchi, P.: Host specificity of abomasal nematodes in free ranging alpine ruminants. Vet. Parasitol. 90:221-230, 2000.