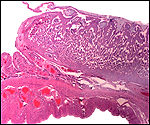
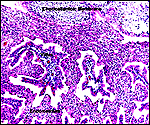
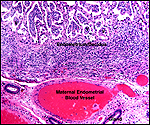
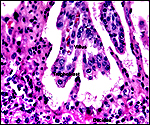
|
(Clicking
on the thumbnail images below will launch a new window and a larger
version of the thumbnail.)
|
Gazella leptoceros leptoceros
Order: Artiodactyla
Family: Bovidae
1) General Zoological Data
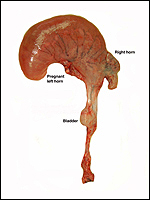
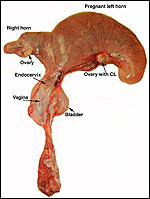
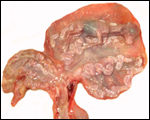
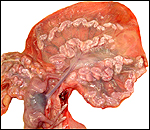
Slender-horned gazelles ("Dunengazellen" in German, also known as "Rhime" and Loder's gazelles) are now rare, endangered gazelles that once ranged in the desert from Algeria to Egypt (Saleh, 1987). Although paler and slightly larger, this species can be mistaken for Dorcas gazelles (Newby, 1984; see also my chapter on Dorcas gazelles). Groves (1969) found greater similarity to the goitered gazelle in his meticulous comparative skeletal study, and hybridization between the two forms has been described. Distinguishing features are the horns. The slender-horned gazelles are very small animals, weighing 14-18 kg and they have been maintained in only a few zoos. Those animals come from a very small founder stock of individuals (Thomas et al., 1986). For this reason, inbreeding is a problem for the captive population. At the San Diego, as in some other locations, numerous births have occurred. The longevity of slender-horned gazelles is about 11 years, and age of first parturition is at about 11-12 months (Mentis, 1972).
The evolutionary history of Bovidae and of also that of many gazelles is still controversial. It was addressed with modern DNA studies by Gatesy et al. (1997), and also by Matthee & Davis (2001). The chromosomal study by Effron et al. (1976) suggested that, because of the autosome/X fusion of this and many other African gazelles, they have a common ancestor, despite their current great variability in phenotypes and karyotypes.
There have been suggestions in the scientific literature that one should distinguish two, perhaps three subspecies of slender-horned gazelles: G. l. leptoceros, G. l. loderi, and perhaps G. marica. Detailed studies of these putative relationships may be found in the study conducted by Lange (1972).