|
(Clicking on the
thumbnail images below will launch a new window and a larger version
of the thumbnail.)
|
| Last updated: |
| February 16, 2005. |
Urial Sheep
Ovis vignei
Order: Artiodactyla
Family: Bovidae
1)
General Zoological Data
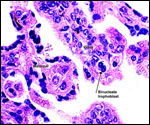
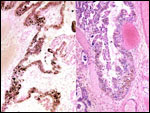
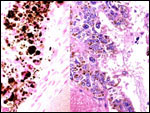
The principal reason for adding this chapter on urial sheep to the more general chapter on domestic sheep is first, the much different genetic background of urial cytogenetics, second, that it gives me a chance to detail my studies of the large quantity of yellow pigment found in the trophoblast of most ruminant placentas, and finally, that urial are becoming very endangered and the possibility of future cloning should be contemplated. For that reason, knowledge of placentation may be useful. The reader should also make reference to the earlier general chapter on sheep placentation.
The name Urial derives from the Punjabi, denoting a Himalayan wild sheep (Gotch, 1979). The animals originate from India , Kazakhstan and neighboring countries (Nowak, 1999). They are now an endangered species and are said to hybridize with Ovis orientalis in a narrow region of northern Iran (Nadler et al., 1971; Valdez et al., 1978). There is much controversy in general, however, on the origin of the many different types of sheep, and the numerous different cytogenetic findings also suggest that interbreeding among ovine species occurs (Gray, 1972). Robertsonian fusion mechanisms of chromosomes are largely responsible for this speciation, as was shown i.a. by Bunch et al. (1976). They considered that a “prezygotic selection” was the mechanism of chromosome reduction from an ancestral stock with 2n=60. Hiendleder et al. (1998) found by mtDNA study of sheep that the origin of domestic sheep was from two different forms of wild sheep that did not include the urial or argali sheep. Adult male urials weigh around 60 kg.
The domestic sheep, however, is generally presumed to have derived from Ovis musimon , the mouflon, and a species that is now distributed across Mediterranean islands. Domestication is thought to have begun approximately 10,000 years ago. There are six well-defined species and numerous subspecies of sheep distributed throughout Asia and North America . The domestic sheep now occurs worldwide and this is partly the reason for the dwindling populations of wild sheep - because of hybridization. Because some sheep species and/or subspecies are significantly endangered, they now require protection and have been listed as CITES I species, as is the urial, O. vignei (Nowak, 1999). Longevity varies with species type and with race. For mouflons, the maximal life span is given as 19-20 years (Puschmann, 1989). Jones (1993) gives a maximal life span for urials as 13 years 10 months.
 |
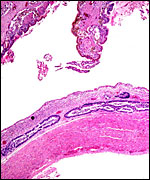
Transcaspian urial sheep at San Diego Zoo. |
 |
Transcaspian urial at San Diego Zoo. |
2) General gestational data
Keisler (1999) has provided a detailed review of the reproductive parameters of domestic sheep and goats, but much less information is available on urials. There are about 800 different "breeds" of domestic sheep, with many different physical characteristics. Depending on the breed, puberty occurs between 5 and 12 months. Breeding occurs throughout the year in domestic sheep and is somewhat dependent on photoperiods. The duration of estrus is between 3 and 72 hrs. (average 29 hrs.); it is influenced by the presence of a male. The estrous cycle averages 16 days.
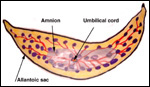
While the length of gestation in wild sheep species varies between 150 and 180 days, the domestic sheep has an average length of gestation of 148 days. Brown (1936) gave the length of gestation for urials as approximately 6 months. Most sheep produce singletons, but some races regularly have twins, even triplets. This certainly is true of urials (Zuckerman, 1953). Singletons weigh about 1.5 kg (Alexander, 1964).
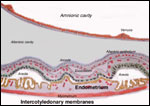
The placental weight is difficult to ascertain, as the membranes are voluminous and much of the structure is of little importance to fetal growth. Kleemann et al. (2001) provided weights of around 600 g for domestic sheep. These authors were interested in the relation of placental and fetal sizes after progesterone treatment in early gestation. Whether these weights reflect a direct relation to the provision of nutrients to the fetus can be questioned. It is for that reason that investigators have weighed what they considered to be the more meaningful weight - that of blotted cotyledons. This is discussed in detail subsequently for domestic sheep (Alexander, 1964).
3) Implantation
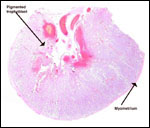
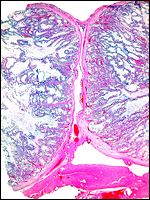
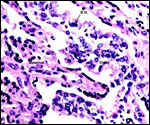
Within three to four days, the fertilized domestic sheep ovum reaches the uterus as a morula. The blastocyst develops by day 6 and sheds its zona pellucida by day 8 or 9. The blastocyst enlarges rapidly after the tenth day (Assheton, 1906). It then becomes "sticky" and, on about day 17-18, it attaches with cytoplasmic protrusions to the endometrial surface. Naturally, this attachment is only on the caruncles of the endometrium. The placenta remains diffuse for the first month or so after its mesometrial attachment (King et al., 1982). Lawn et al. (1969) did an electronmicroscopic study of goat and domestic sheep placentas. Their youngest sheep gestation was at 42 days of pregnancy; the cotyledons were then just beginning to develop their villous structures. The trophoblast was apposed to an intact endometrium that had the appearance of producing a protein secretion. Microvilli were abundant and occasional binucleated trophoblastic cells were present. Interdigitation of the cells progressed thereafter and the endometrial epithelium began to transform. Moreover, multinucleated giant cells became apparent. The syncytial cytoplasm then acquired characteristic cytoplasmic granules.
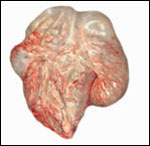 |
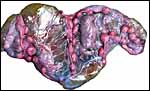
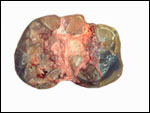
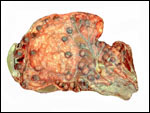
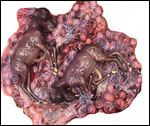
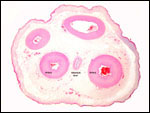
Uterus of urial containing mid-gestation twins, one in each uterine horn. |